PBLD: Neuraxial Blockade and Issues Around Consent, Coagulation Conundrum

This problem-based learning discussion (PBLD) is based on clinical dilemmas encountered on a daily basis in the cardiothoracic intensive care unit (ICU). The PBLD offers the perspective of physician providers in the United States, Canada, and United Kingdom and on Twitter. The stem and questions were created by Drs Ip, Mariano, and Schroeder. Dr. Mariano then shared select questions via the ASRA Twitter account (@ASRA_Society) for ASRA members at large to share their opinions.
A 23-year-old man was sedated and intubated in the cardiothoracic ICU following a double lung transplant for his cystic fibrosis. He was admitted postoperatively into ICU at 5 am, and you were called at 9 am to insert an epidural so the ICU team could extubate the patient. They did a set of routine blood tests on admission to the ICU, and the results were as follows:
- International normalized ratio (INR) = 1.2 (0.8–1.2)
- Activated partial thromboplastin time (PTT) = 45 seconds (27–39 seconds)
- Platelet count = 200 × 109/L (140–450 × 109/L)
When you arrived at the ICU, you found that the patient was sedated on propofol infusion, and he was not consented for an epidural preoperatively.
Would You Perform an Epidural on This Patient?
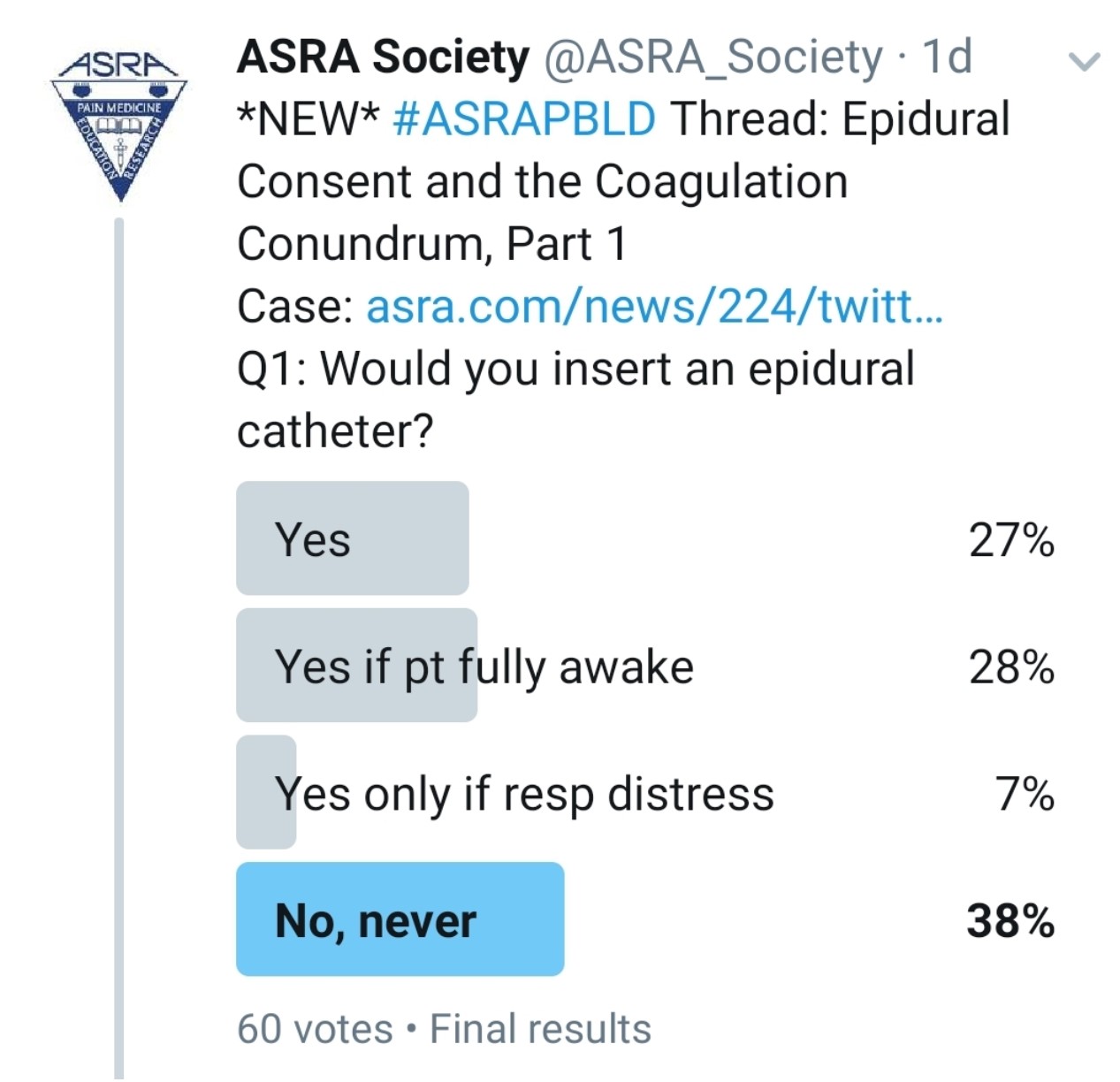
Kwofie: Although we do not do lung transplants in our institution, this scenario is analogous to many cases that we see in our practice, and I feel that the principles are similar. My first course of action in all patients is to start with a history, thorough physical examination, and review of labs and imaging.
My history typically includes a review of all surgeries, comorbidities, and medications, but here I would also focus particularly on several key elements:
- The nature of the surgery and location of incision
- Previous surgical history and any previous perioperative pain issues
- Chronic pain and addiction history
- Problems or successes with regional anesthesia in the past
- Recent and current pain status (pain scores at rest and with activity)
- Functional assessment (ability to take deep breaths and cough, ability to turn in the bed with a nurse’s assistance)
- History of spine problems (eg, fractures, surgery, scoliosis, neurologic issues)
- History of coagulopathy or anticoagulants (including dose and timing).
- History of this transplant surgery (bleeding, transfusion, need for cardiopulmonary bypass)
- Continuing indications for intubation (oxygenation, ventilation, pulmonary toilet, level of consciousness, delirium, presence of leaks in the bronchopulmonary tree, cardiovascular support requirements)
- Presence of exacerbating factors (eg, chest tubes) and any anticipated timeline for their discontinuation, if known
I certainly care about the patient’s overall comfort, but the largest potential contribution from a thoracic epidural would be to facilitate sustained extubation and to optimize postextubation ventilation status.
With respect to the coagulation status, I would review the surgery for blood loss, fluids administered, and transfusion to anticipate the potential complexity of a perioperative coagulopathy. If the patient had a large-volume blood loss or transfusion, I would likely want to see a fibrinogen level. I am also concerned not only about the patient’s present coagulation status but also where it is going, because any epidural will eventually have to come out and require sustained favorable coagulation.
The INR is at the upper limit of normal and the platelet count is also normal, but the PTT is slightly elevated. In isolation the PTT is of unknown clinical consequence, so I would investigate further. Could it be residual heparin effect? I would want to know the history of the patient’s PTT values. Presumably, he has past PTT results from a healthy state, and I would want to know if it was normal or elevated. If his previous PTT had been elevated, then I would want to know if he had a Dade PTT and a 50:50 mix. If a 50:50 mix corrects it or the Dade PTT is elevated, it suggests impairment in factor quality or quantity. The challenge of an elevated PTT is that PTT may remain normal until factor levels are very low, so the small magnitude of impairment in PTT is not reassuring. If my evaluation demonstrated abnormal coagulation studies, I would not offer him an epidural at this time.
Gray: I would defer epidural placement at this time because of the patient’s sedation and lack of informed consent.
Grant: To perform an epidural, I would need to obtain a history, physical exam, and consent. The case presentation provides some of the history, but I want to know what previous anticoagulants he received (if any), how much blood loss occurred during the surgery, his current temperature, and whether he has any intraoperative coagulation data. Is he awake enough to consent, or was he consented before the case?
Pawa: For me, the simple answer to this question with the information presented is no, I would not perform the epidural. In the United Kingdom, the results of a legal case known as the Montgomery ruling or judgment[1],[2] have a significant impact on the way we consent for medical procedures, and in the absence of more information, without the preprocedure consent, I would opt to abide by my Hippocratic oath and “first, do no harm.”
How Do You Consent This Patient?
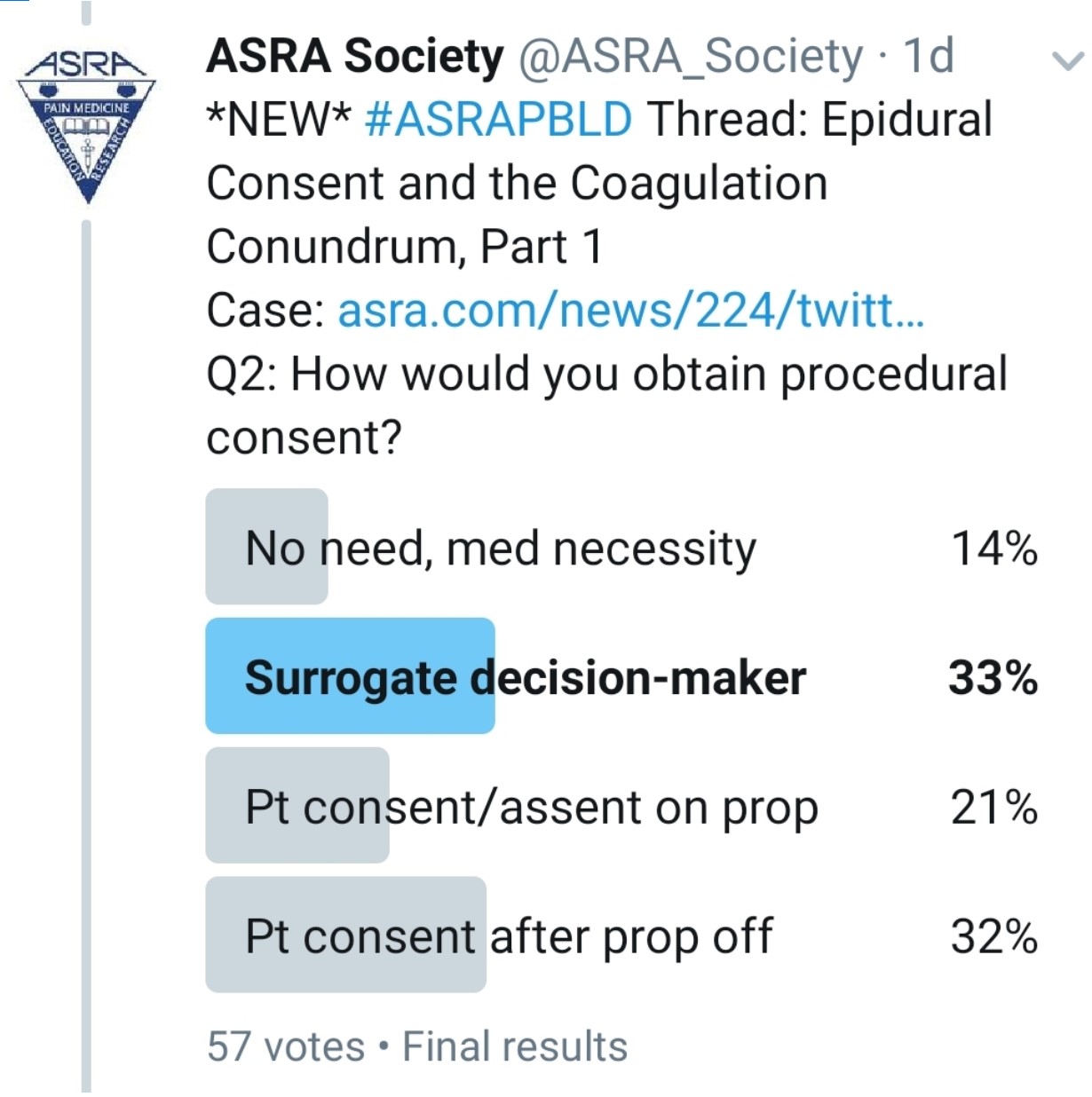 Kwofie: As a component of the preoperative assessment process, the anesthesiologist can describe the risks, benefits, and alternatives to thoracic epidural anesthesia and obtain an informed consent before the patient goes to the operating room. In the case of an institution that routinely performs postoperative epidurals in lung transplant recipients, such a routine for informed consent might be ideal. This did not happen in this case. Lack of preoperative consent is a challenging situation and not ideal. The challenge is that the patient might be competent but is likely iatrogenically impaired by his sedation medication. When I encounter such a situation, my routine is to involve both the patient and the substitute decision maker in a discussion of the risks and benefits of a procedure as much as possible. My hope would be to have consensus between the patient and the substitute decision maker before I proceed.
Kwofie: As a component of the preoperative assessment process, the anesthesiologist can describe the risks, benefits, and alternatives to thoracic epidural anesthesia and obtain an informed consent before the patient goes to the operating room. In the case of an institution that routinely performs postoperative epidurals in lung transplant recipients, such a routine for informed consent might be ideal. This did not happen in this case. Lack of preoperative consent is a challenging situation and not ideal. The challenge is that the patient might be competent but is likely iatrogenically impaired by his sedation medication. When I encounter such a situation, my routine is to involve both the patient and the substitute decision maker in a discussion of the risks and benefits of a procedure as much as possible. My hope would be to have consensus between the patient and the substitute decision maker before I proceed.
Gray: I would wait until the presented issues can be addressed and then discuss risks and benefits with the patient.
Grant: The best plan of course is to discuss consent with the patient and their family prior to the case. If the patient is awake, then I would discuss consent in the unit if possible. If consent has not been obtained, I am very cautious about placing an epidural. This is a systems issue. The best plan is to meet with the transplant team and standardize goals and patient education prior to surgery.
Pawa: I think this is a really tricky one because we all have a desire to alleviate pain and discomfort. If I follow my stance, the only option I have is to wait for the patient to be awake and responsive, with the propofol off, so that he displays capacity to provide consent. I would not delegate to a surrogate decision maker or gain consent for a thoracic epidural in this instance.
Would It Be Better to Do a Preoperative Epidural?
Kwofie: The risk of inserting a preoperative epidural is largely related to the potential for coagulopathy and bleeding. A cardiopulmonary bypass may be necessary, which itself can cause significant coagulopathy in addition to the large doses of heparin that are required. Furthermore, the potential for large-volume bleeding during the operation can also lead to a complex coagulopathy.
The other consideration is that if surgical complications arise, the patient might not be able to be extubated for several days. If that occurs, the patient would have an epidural in situ that may pose an infection risk. Although the risk is small, it increases incrementally each day, so the patient is arguable receiving the highest risk with the least functional benefit.
The benefit of preoperative epidural placement is that it is often easier to properly position the patient, which may make the procedure less technically challenging: I never try to sit intubated patients but rather do the procedures in the lateral position. In addition, obtaining cooperation from an intubated, postoperative patient may require generous sedation, which could limit necessary feedback from the patient during the procedure (eg, reporting paresthesia). When I encounter these situations, I routinely use ultrasound scout imaging of the spine to confirm the location of the lamina and interlaminar spaces. The sonographic assessment’s anatomical guidance adds a level of safety, especially when patient positioning may be difficult.
However, I do recognize that I am cautious. Several institutions have published their experience with thoracic epidurals in patients before cardiopulmonary bypass, and the use of spinal drains in thoracic aneurysm surgery is common, but complications may be underreported.[3],[4]
Time may demonstrate that my caution is overstated; on balance, patients likely have less risk and more benefit with an epidural placed postoperatively at a targeted time point to facilitate extubation, if at all, rather than placed preoperatively, as long as the physician accepts that the procedure may be a bit more challenging, requiring ultrasound and occasionally deep sedation.
Gray: Yes, I find this works much better for a number of reasons (eg, wakefulness, cooperation, positioning, sterile conditions).
Grant: Preoperative placement sounds good, but time pressures before lung transplant often make this difficult. Epidural administration of local anesthetics may also produce intraoperative hypotension. The other issue is what to do if the tap is bloody?
Pawa: Placement of an epidural preoperatively is ideal but may well be complicated if cardiopulmonary bypass/extracorporeal membrane oxygenation and full heparin anticoagulation were used. Although some evidence supports the benefit of preoperative placement,[5].[6] failing that, preoperative, fully informed consent for a postoperative epidural is an alternative option.
What Would You Do if the Patient Had a Traumatic “Bloody” Tap During the Preoperative Epidural Placement?
Kwofie: The risk of epidural hematoma in patients who have a traumatic tap during placement may be higher, particularly in those who will receive large doses of heparin. However, I would already be unwilling to place an epidural in a patient who might require cardiopulmonary bypass, even before the additional risk of a traumatic tap.
Gray: Presumably traumatic tap means blood observed from the needle or catheter during placement. If the catheter is functioning well (negative test dose), I would manage the epidural catheter per the usual routine. There is no need for serial neurologic checks, given the mild abnormalities of coagulation.
Grant: The time from the traumatic tap to bypass is the issue. Very often, a couple of hours pass between the traumatic tap and anticoagulation. This is much longer than the normal bleeding time, and clot formation should have occurred prior to anticoagulant administration. You can proceed with the case.
Pawa: Using the well-known guidelines or the ASRA Coags app as a guide, the answer would be to delay surgery for 24 hours,[7] which has significant implications.
The Case Continues
The patient has been extubated and is now awake but in a lot of pain; he finds coughing difficult and is unable to take deep breaths. You were asked to review his analgesic options again.
You Realized That He Was Given Heparin Subcutaneously 15 Minutes Ago. Would You Do an Epidural?
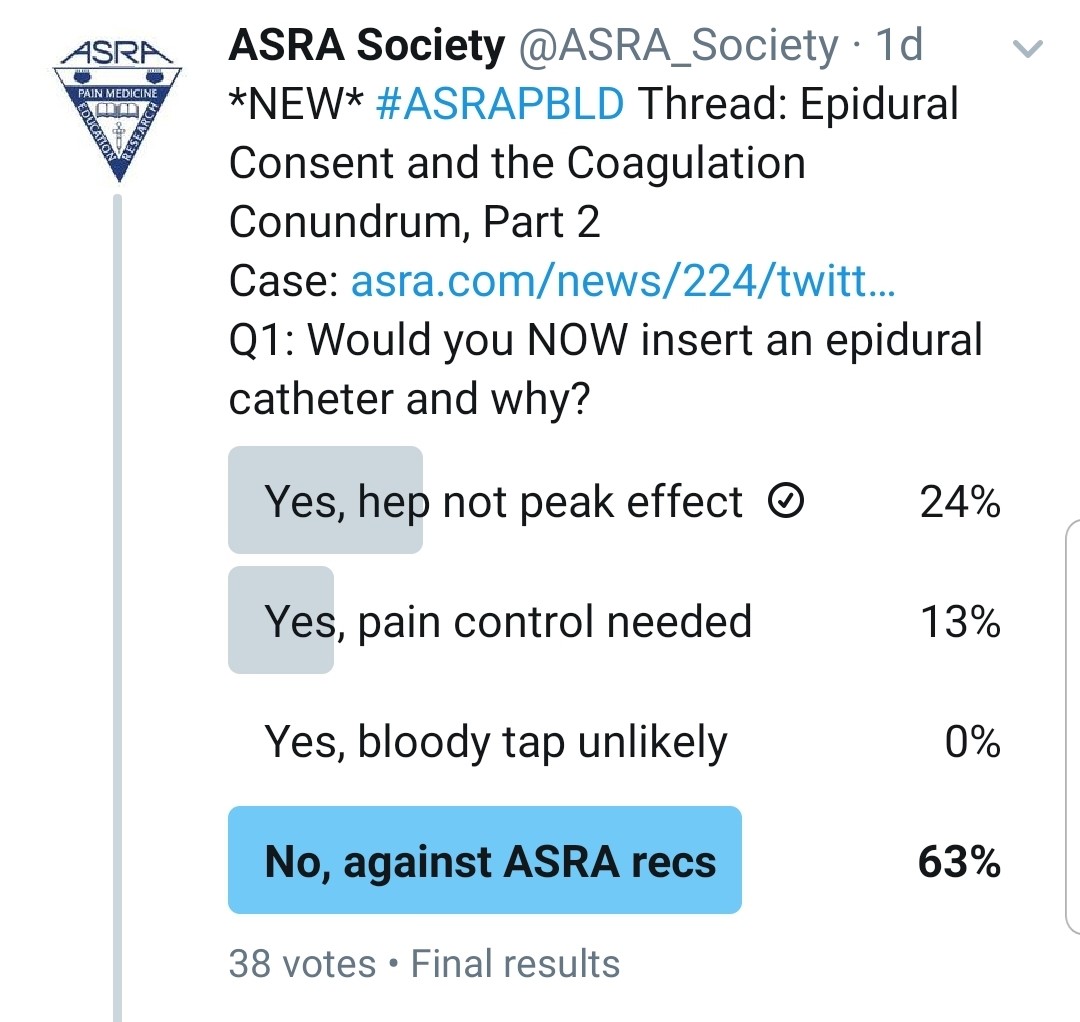 Kwofie: Occasionally, a subcutaneous dose may be inadvertently administered intravenously or have a more rapid and dramatic anticoagulant effect than intended. For this reason, I would prefer to wait for the heparin effect to diminish.
Kwofie: Occasionally, a subcutaneous dose may be inadvertently administered intravenously or have a more rapid and dramatic anticoagulant effect than intended. For this reason, I would prefer to wait for the heparin effect to diminish.
Gray: If the heparin dose was 5,000 units of subcutaneous unfractionated heparin (for low-dose prophylaxis), I would wait 4 to 6 hours prior to epidural placement per the most recent ASRA anticoagulation guidelines.[7] I do not routinely check PTT prior to epidural placement, even though cases of high PTT values after heparin have been observed.[8]
Grant: I refer to the ASRA Coags app on my phone and follow the current guidelines. What kind of heparin was administered and what dose? If it is unfractionated, you can order a new coagulation test, but I would wait the recommended 4 to 6 hours[7] prior to placing an epidural.
Pawa: I would not insert an epidural immediately. I would determine exactly what type of heparin (low molecular versus unfractionated) given and the dose, before making a decision on timing of subsequent placement.
What if He Received Subcutaneous Heparin an Hour Ago?
Kwofie: Subcutaneous heparin demonstrates peak coagulation effects at about 2 to 3 hours. Also, as stated previously, some patient-administered subcutaneous heparin can have therapeutic levels of heparin within the first 2 hours. In this case, I would prefer to wait for the heparin effect to diminish.
Gray: Same answer as before: wait 4 to 6 hours from the time of dose.
Grant: I would still wait. If I was really pressured and the patient was deteriorating, I would order a coagulation screen. The recommendation is to wait the recommended 4 to 6 hours.[7]
Pawa: Definitely not. Even if it was unfractionated heparin, the anticoagulant effect would persist for 4 to 6 hours.[7]
What Other Analgesic Options Does the Patient Have? Would an Interfascial Plane Block Be an Appropriate Alternative?
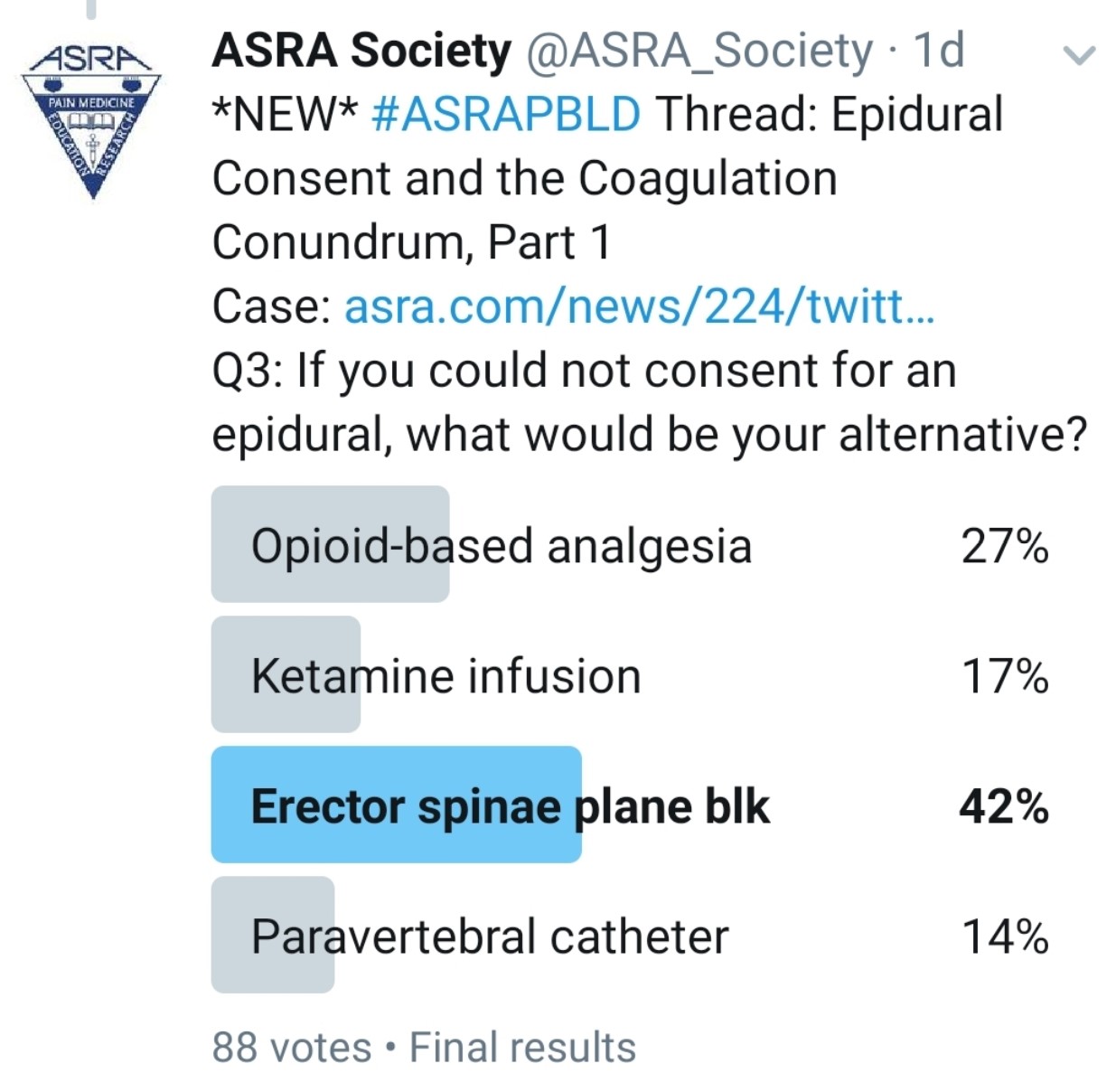
Kwofie: Bilateral paravertebral catheters or single-shot blocks may be helpful, but their effect is limited to about five levels of spread per side and high volumes of local anesthetic because of the need for two catheters. Also, they may have a risk of bleeding into a noncompressible space.
An immense variety of recently described chest wall blocks might be beneficial for sternotomy pain, including midpoint to transverse process, pectointercostal fascial, serratus plane, erector spinae plane (ESP), retrolaminar, and rhomboid intercostal and subserratus (RISS) plane block. Evidence is inadequate to support one technique over another for efficacy or safety. Also, all of these techniques would have to be done on both sides to be effective and would require large doses of local anesthetic.
Gray: Although I commonly use interfascial plane blocks, I don’t think these would be effective for a double lung transplant. I might consider ESP catheters in some patients for less extensive surgery, but this is a relatively new technique, and we currently lack prospective, randomized studies showing benefits to patients. However, some case reports and case series have been encouraging.
Grant: Bilateral ESP block catheters might be an option. ESP is a superficial block, but the pleura is still visible on the screen. In addition, with ESP blocks, local anesthetic has been observed entering the paravertebral and the epidural space. If local anesthetic can enter the epidural space, then blood may enter the same locations and potentially result in a hematoma.
Pawa: Multiple nonopioid analgesic options are available. Thoracic paravertebral blockade requires the same adherence to anticoagulation guidelines as epidural placement and is therefore not ideal. Interfascial plane blocks present really useful alternatives. I would consider the ESP block, the retrolaminar block, RISS, or the serratus anterior plane block.
Clinically, Do You Find Yourself Doing Fewer Epidural and More Interfascial Plane Blocks? Are They Comparable?
Kwofie: My clinical experience with chest wall interfascial plane blocks is that they can be unreliable. On occasion, what looks like a very good spread of local anesthetic on ultrasound does not always lead to a demonstrable sensory level or a significant clinical improvement for the patient. The literature suggests that thoracic epidurals are also unreliable and that the failure rate for both procedures might be as high as 30% or greater.[9] I routinely use an electrical stimulation test (Tsui test) for thoracic epidurals to assess the level and position of the catheter immediately after placement to maximize efficacy.
In my experience, interfascial blocks are generally inferior to thoracic epidurals in terms of analgesic efficacy. However, not all patients meet the stringent coagulation and anatomic requirements nor accept an epidural’s associated risk, but they might be amenable to and appropriate for a fascial plane technique, which carries much less risk and are relatively simple to manage postoperatively. I look forward to studies that will help us to better estimate the balance of risk and reward from these conflicting techniques.
Gray: Yes, I am increasingly using interfascial plane blocks. In general, they are similar or slightly less effective than epidurals for pain relief, but the side effect profile is better and patient acceptance is high.
Grant: I do find myself using more interfascial plane blocks, but I do not use them as an anesthetic and only use them for postoperative analgesia. I am still trying to assess their use in different clinical locations, patient settings (critical care versus ward), and patient types (opioid naïve versus tolerant).
Pawa: In my clinical practice, I place very few thoracic epidurals. I now use predominantly thoracic paravertebral blocks, ESP blocks, or quadratus lumborum blocks. Depending on the indication, I find them comparable and actually more in line with enhanced recovery protocols. The only place where I find I need thoracic epidurals now is for complex abdominal wall reconstruction cases.
Are All Interfascial Plane Blocks Considered Superficial Blocks and Not Affected by Deranged Coagulations?
Kwofie: I do not believe that these techniques are all the same. I think that recently described approaches for classification of bleeding risk for peripheral nerve blocks is helpful.[10] It classifies a block based on three criteria. The first is the proximity of the needle path to various critical structures. The second is the ability for noninvasive intervention in the presence of bleeding or a hematoma (ie, compressible or noncompressible). The final criterion is the ability to clinically assess the severity of a hematoma or bleeding if present. This approach is useful to help make judgments regarding the multitude of different peripheral nerve blocks. For example, a transmuscular quadratus lumborum block likely does not carry the same risk profile as an ESP block.
Gray: Bleeding risks are very small for most interfascial plane blocks, but they are associated with a number of considerations for bleeding complications (eg, superficial, compressible, observable site). Deeper blocks such as paravertebral blocks or lumbar plexus blocks are treated the same as neuraxial blocks, per ASRA anticoagulation guidelines.[7]
Grant: Every patient and proposed interfascial plane block has its own risks of puncturing a blood vessel and causing a hematoma. For example, quadratus lumborum blocks are in close proximity to the lumbar artery, which may be difficult to visualize on ultrasound imaging. The artery is not easily compressible in this location, and hemorrhage may result in a retroperitoneal hematoma.
Pawa: I don’t think that all interfascial plane blocks can be treated the same. It certainly depends on the precise location, the compressibility of the site, and the individual patient’s anatomy, vasculature, and degree of coagulation derangement. As a general rule, though, I am more likely to use an interfascial plane block with altered coagulation than I am an epidural, assuming a risk-benefit discussion has been completed.
Would You Do an Epidural if the Patient’s INR Is 1.5?
Kwofie: A review of the patient’s laboratory values trends is essential, as well as an assessment of the cause of the laboratory derangement. I would evaluate, as stated earlier, for any other coagulation derangements and anticoagulants. Treatment with vitamin K may improve the INR. The challenge with any altered coagulation is not only the current coagulation status but also the status when the catheter will need to be removed. In this case, I would prefer to use a superficial fascial plane block such as an ESP block or to wait for an improvement in the coagulation status after vitamin K in 24 hours.
Gray: Because the value is on the borderline, I usually use alternatives in this setting after considering the risks and benefits. Other considerations (eg, concomitant medications such as aspirin, platelet function) often play a role in clinical decisions. We also typically want to know in what direction abnormal lab values are trending and why. If the patient has recently been on warfarin, I would not proceed with epidural placement because recovery of vitamin K–dependent clotting factors may not be adequate.
Grant: No, I will stick with the ASRA guideline.
Pawa: I would need a bit more information than this. INR of 1.5 is certainly the upper limit, but it would depend on the context, on how long before the warfarin use (or cause of INR abnormality) had ceased, and whether the patient has any other drugs in his system that may affect coagulation, such as aspirin or other nonsteroidal anti-inflammatory agents.
The Case Continues
As you read through the patient’s most recent vitals and laboratory work, you see that his temperature is 37.3°C and his white blood cell count is 23. The surgeon walked by, and he says all patients’ white blood cell counts are elevated posttransplant.
Would You Insert an Epidural Catheter If He Gave Proper Informed Consent and His Last Heparin Injection Was Now 6 Hours Ago?
Kwofie: Again, I would do a thorough patient assessment. The patient is afebrile but has a mildly elevated white blood cell count. Did he have an active infection before the surgery? Is there a potential source for infection? Has it been treated or not? I would consider placing an epidural if I was satisfied clinically that he did not have an untreated or uncontrolled infection.
Gray: Because the infection risk is very low, especially if the patient is receiving antibiotic therapy that covers skin flora, I would proceed in the case described above. If a sepsis workup is in progress (eg, fever of unknown origin, blood cultures, removing indwelling lines and culturing their tips), then I would not proceed.
Grant: This again is a tough call. A lot of patients do have a high white blood cell count. It is easy enough to reassess the complete blood count and review his temperature to see what the trends are.
Pawa: All of the signs here are pushing me to say “no.” In this case, I would happily use bilateral ESP blocks and feel reassured that I had performed an intervention that was more likely to help than cause harm, but my answer is also based on the confidence I have with placing ESP catheters and the limited number of thoracic epidurals I currently use.
If the Patient Does Have a Post–lung Transplant Epidural, How Would You Manage His Hypotension?
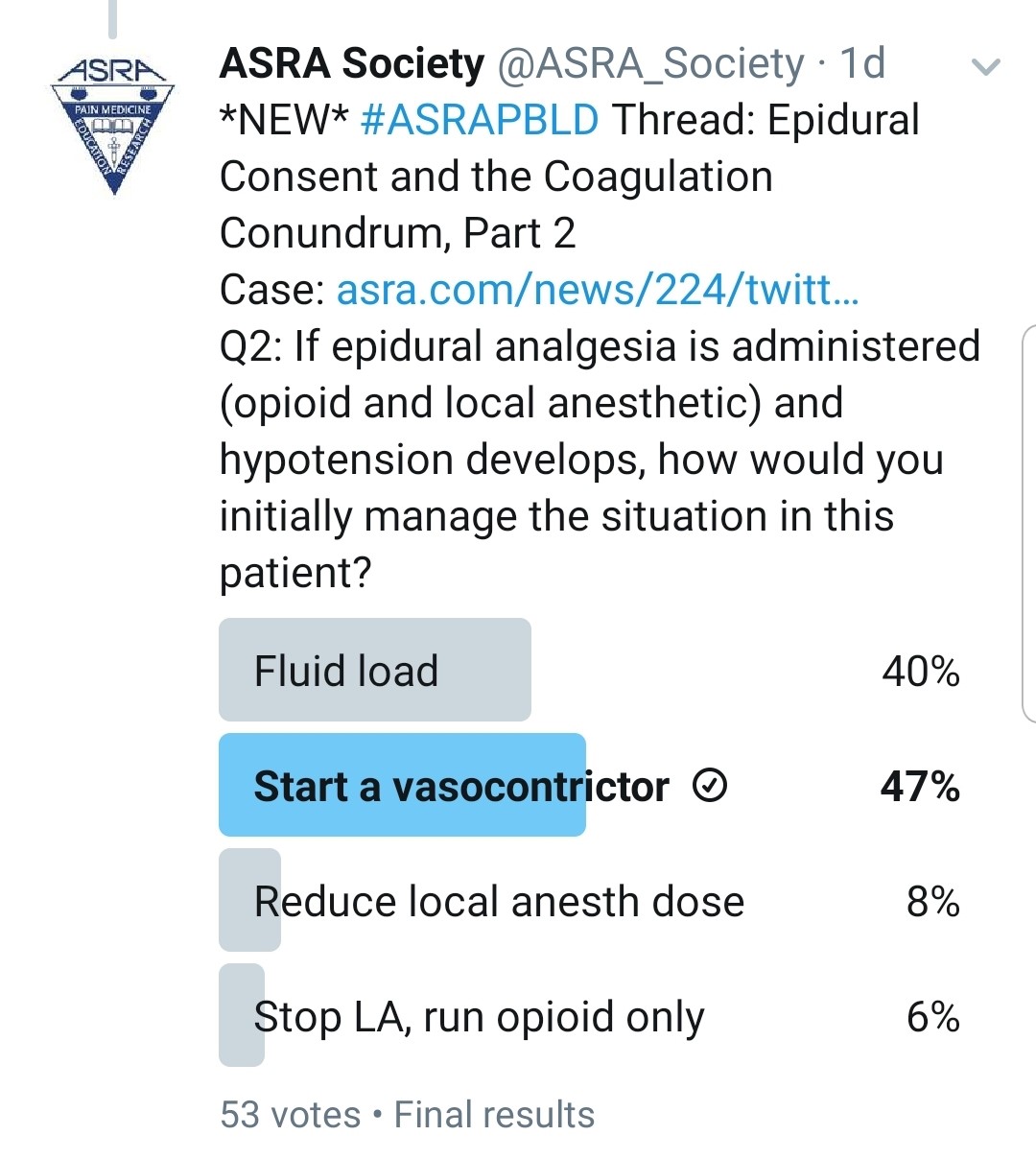
Kwofie: Patients who are still intubated and being actively ventilated have many potential etiologies for hypotension (eg, cardiovascular problems such as undertreated hypervolemia, metabolic derangements, vasodilatation from sedative medications). This may require patients in the early postoperative period to have modest continued vasopressor requirements. Determining which potential problem may be the greatest contributor is sometimes difficult, and overly aggressive fluid resuscitation is undesirable.
Typically, in a patient who has not yet been liberated from the ventilator, I would run a mixture of local anesthetic and opioid initially at a modest dose with the knowledge that it may have some negative impact on blood pressure but will give the best analgesic and ventilation results. I frequently find that once patients are extubated, vasopressors can be weaned even in the presence of a thoracic epidural. The local anesthetic will help to produce the best analgesia to maximize the likelihood of sustained liberation from the ventilator.
Once the patient is extubated and otherwise stable, if I cannot successfully wean vasopressor therapy, I would consider converting the epidural infusion to opioid only.
Gray: I would use a low epidural infusion rate with a low concentration of local anesthetic and consider a pure opioid infusion via the epidural catheter. These strategies are only slightly effective, however. This patient will be managed with minimal fluids in the ICU, so this is a common problem after lung surgery.
Grant: Chronically, at the first sign of hypotension, our epidurals are stopped. We try to work with the unit to start a vasopressor if the patient is still in the ICU. If the patient is on the ward, we reduce the local anesthetic concentration or reduce the infusion rate. If that fails, we remove the local anesthetic from the solution and just run an epidural opioid infusion.
Pawa: The challenge here is to achieve analgesic benefit with limited deleterious effects of the sympathetic block. I would aim to use the most dilute concentration of local anesthetic available, use a baseline rate with minimal bolus dose, ensure optimal volemic status, and, if those options fail, use a small amount of vasopressor. My impression is that this would be a nonissue if an ESP catheter was placed.
Do You Avoid High Thoracic Epidurals in Older Patients Because of the Risk for Acute Renal Failure From Hypotension?
Kwofie: No. In patients whom I feel are at particular risk because of hypotension, I would either increase their level of monitoring or change their epidural mixture to an opioid only. However, older patients are more likely to be on anticoagulants that need to be managed preoperatively. Also, they are more likely to present anatomic challenges during insertion, making the procedure more technically difficult.
Gray: No, because the alternatives are typically worse.
Grant: Not always. Reduced dosing and concentration of local anesthetic are key for older patients. Also important is acute management of any epidural issues. If all else fails, consider one of the interfascial plane options.
Pawa: Not necessarily. Again, it would depend very much on the indication, comorbidities, and other risk factors. I never say “never” to anything, but we have alternatives now.
If the Patient Has a History of Multiple Sclerosis, Would You Perform an Epidural?
Kwofie: Multiple sclerosis (MS) is classically described as a demyelinating disease of the central nervous system. However, we know that it also affects demyelination of the peripheral nervous system. Although patients may be more likely to have a nerve injury or flair of their MS after an epidural, demonstrating causal effects has been difficult. Many have reported successful cases of epidurals for managing pain in patients with MS, but a theoretic additional “double crush” risk still exists.
I start by doing a thorough history and physical examination, including a neurologic examination to identify any preexisting fixed or evolving neurologic deficits. I usually discuss both the known and unknown risks with patients or their surrogate decision makers and decide on a patient-specific plan. Typically, in the absence of evolving severe neurologic deficits, I usually counsel patients that risks are low but to some degree unknown; however, if we expect substantial benefit relative to alternatives, then an epidural is likely a reasonable choice, and in most cases, I would offer it.
Gray: Yes, but I would limit the dose and concentration of local anesthetic, as recommended by the ASRA practice advisory on neurologic complications.[11] Also, I might abandon the procedure if an inadvertent dural puncture occurred or further reduce the dose of local anesthetic drug if the epidural catheter is eventually placed.
Grant: In this case, I would ensure a good discussion of risks and benefits with the patient and his family and that the discussion was documented. I would perform the epidural with the right patient who was well informed. Other options include more distal blocks (eg, ESP, serratus anterior plane block).
Pawa: If we are talking about the same patient with the rest of preceding potential conflicting reasons presented in this case, definitely no. In another isolated case, I would have a detailed and in-depth discussion with the patient, but I suspect the answer would still be no.
The ASRA News welcomes the submission of topics/cases for consideration as PBLD topics. Please contact ASRAEditor@asra.com with any potential content or author suggestions.
References
- Montgomery v Lanarkshire Health Board. UKSC (Scotland) 11 (2015). https://www.supremecourt.uk/cases/docs/uksc-2013-0136-judgment.pdf
- Chan SW, Tulloch E, Cooper ES, Smith A, Wojcik W, Norman JE. Montgomery and informed consent: where are we now? BMJ. 2017;357:j2224. https://doi.org/10.1136/bmj.j2224
- Guay J, Kopp S. Epidural analgesia for adults undergoing cardiac surgery with or without cardiopulmonary bypass. Cochrane Database Syst Rev. 2019;3:CD006715. https://doi.org/10.1002/14651858.CD006715.pub3
- Cheung AT, Pochettino A, Guvakov DV, Weiss SJ, Shanmugan S, Bavaria JE. Safety of lumbar drains in thoracic aortic operations performed with extracorporeal circulation. Ann Thorac Surg. 2003;76(4):1190–1196.
- McLean SR, von Homeyer P, Cheng A, et al. Assessing the benefits of preoperative thoracic epidural placement for lung transplantation. J Cardiothorac Vasc Anesth. 2018;32:2654–2661. https://doi.org/10.1053/j.jvca.2018.04.002
- Gelzinis TA. An update on postoperative analgesia following lung transplantation. J Cardiothorac Vasc Anesth. 2018;32:2662–2664. https://doi.org/10.1053/j.jvca.2018.05.014
- Horlocker TT, Vandermeuelen E, Kopp SL. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med. 2018;43(3):263–309. https://doi.org/10.1097/AAP.0000000000000763
- Fiebig EW, Jones M, Logan A, Wang CS, Lewis B. Unexpectedly high PTT values after low-dose heparin prophylaxis. Arch Intern Med. 2011;171(7):702–703. https://doi.org/10.1001/archinternmed.2011.106
- Kwofie MK, Launcelott G, Tsui BCH. Determination of thoracic epidural catheter placement: electrical epidural stimulation (Tsui test) is simple, effective, and under-utilized. Can J Anaesth. 2019;66(4):360–364. https://doi.org/10.1007/s12630-019-01302-1
- Tsui BCH. A systematic approach to scoring bleeding risk in regional anesthesia procedures. J Clin Anesth. 2018;49:69–70. https://doi.org/10.1016/j.jclinane.2018.06.011
- Neal JM1, Barrington MJ, Brull R, et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: executive summary 2015. Reg Anesth Pain Med. 2015;40(5):401–430. https://doi.org/10.1097/AAP.0000000000000286