How I Do It: Regional Anesthesia for Breast Surgery
Breast surgery is commonly performed for a variety of indications (eg, abscess drainage, benign lump removal, cosmesis). However, a common indication for breast surgery in a vulnerable patient population is for breast cancer excision. Therefore, this article will focus on the provision of anesthesia and analgesia for oncologic breast surgery.
Physicians must have a significant understanding of the types of breast surgery, correlating innervation, and RA technique efficacy.
Breast cancer is the most commonly diagnosed cancer in women, and standard surgical therapy has undergone a number of substantial changes in recent years. The once-popular radical mastectomy is now rarely used. Newer techniques include the modified radical mastectomy, simple mastectomy, and sentinel lymph node biopsy (SLNB), described in Table 1.[1] When considering regional anesthetic (RA) techniques for breast surgery, physicians must have a significant understanding of the types of breast surgery, correlating innervation, and RA technique efficacy (Table 2) to tailor a patient-specific analgesic and anesthetic plan.
Table 1: Breast surgeries and their regional anesthesia implications
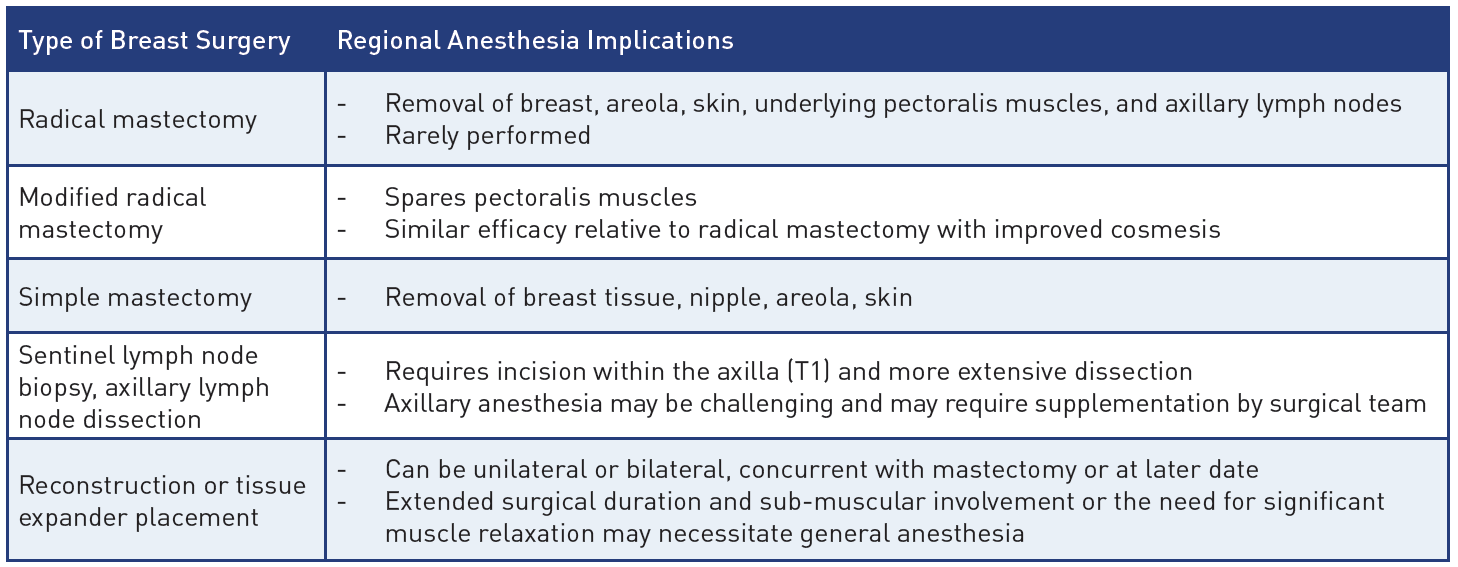
Table 2: Differences between regional anesthesia techniques for breast surgery
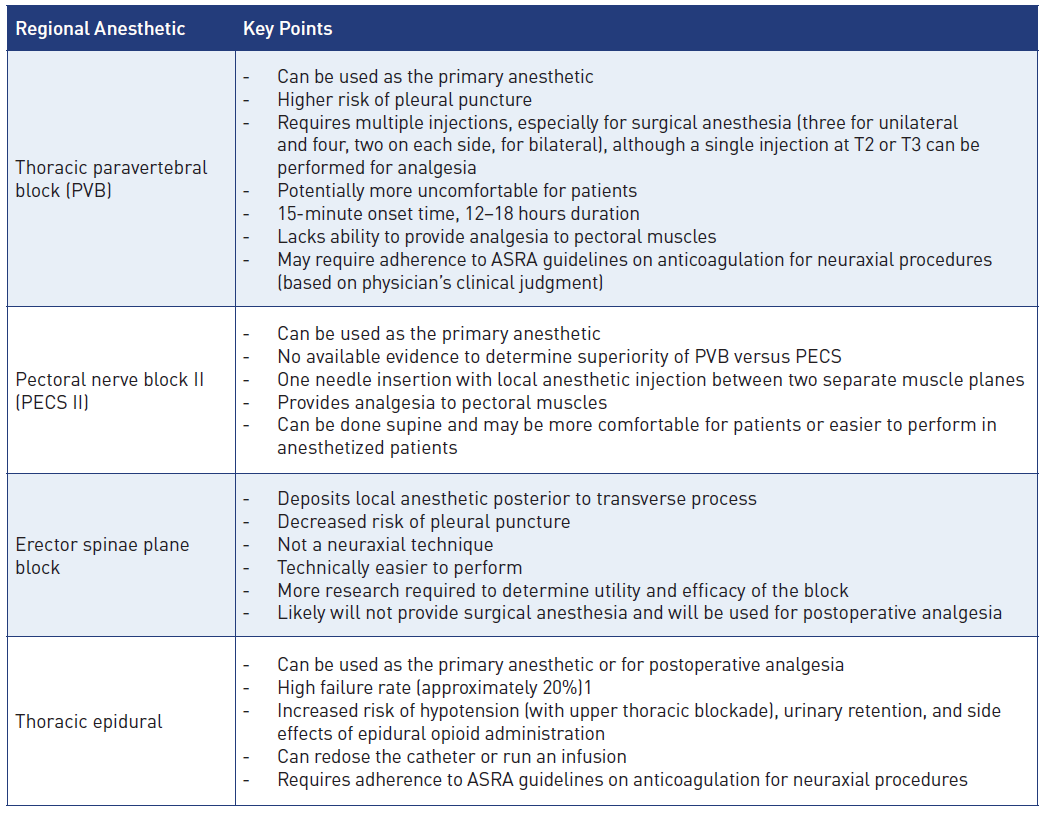
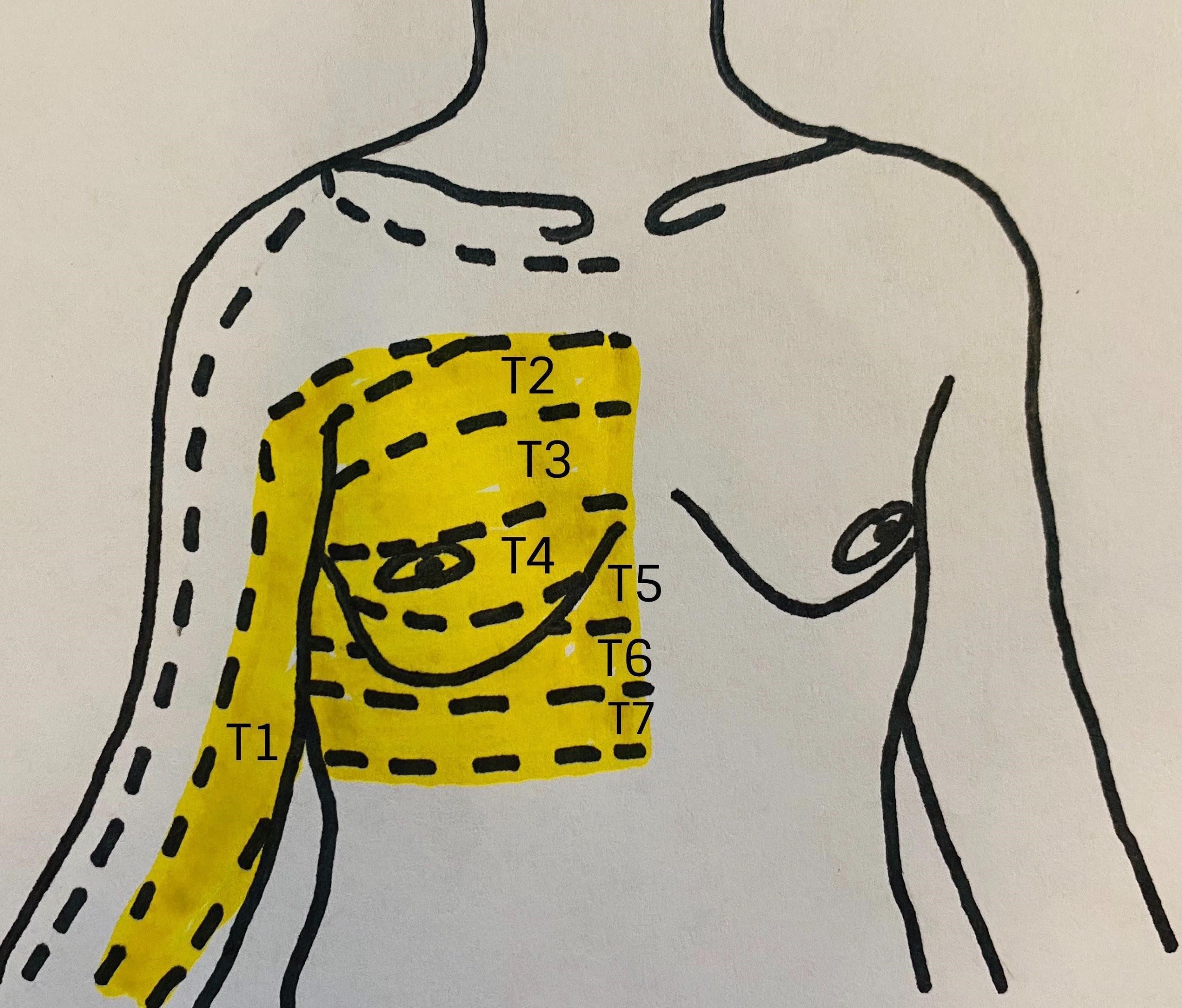
Figure 1: Relevant dermatomes of the breast
Image courtesy of Steven Ethier, DO.
Breast Innervation Simplified
The innervation of the is complex. Branches of the cervical spinal cord and brachial plexus give way to the supraclavicular nerves (C3–C4) as well as the lateral (C5–C7) and medial (C8–T1) pectoral nerves. Those nerves innervate the infraclavicular region and the pectoral muscles, respectively. In the thoracic region, the anterior rami of the spinal nerves give way to the corresponding intercostal nerves supplying their respective dermatome (Figure 1). The intercostobrachial nerve (T2) typically innervates the axilla, whereas T2–T6 innervate the anterior and lateral chest wall.[2]
A variety of RA techniques may provide effective analgesia for breast surgery. The options highlighted in Figure 2 and Table 2 include paravertebral blockade (PVB), erector spinae plane block (ESPB), and pectoral nerve block (PECS II). Epidural analgesia and serratus plane blocks are additional techniques for effective breast analgesia.
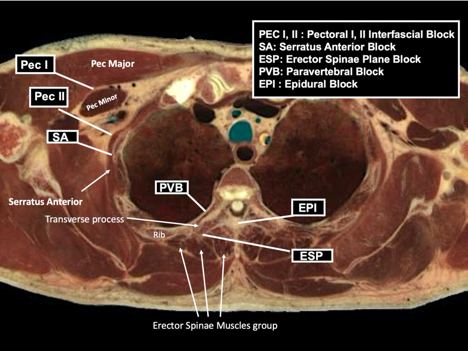
Figure 2: Anatomic locations of common regional and neuraxial blocks used for breast surgery
Reprinted with permission from EPFL, Visible Human Visualization Software, http://visiblehuman.epfl.ch and Gold Standard Multimedia (GSM) http://www.gsm.org.
Thoracic Paravertebral Blockade
Thoracic PVB provides analgesia by blocking transmission at the level of the spinal nerves, attaining unilateral and segmental block of the somatic and sympathetic systems. The paravertebral space is surrounded by the vertebral body and disc as a base, parietal pleura anterolaterally, transverse process and superior costotransverse ligament posteriorly, and endothoracic fascia between the ligament and pleura.3 The thoracic paravertebral space communicates with the epidural space medially and with the intercostal space laterally.1,3
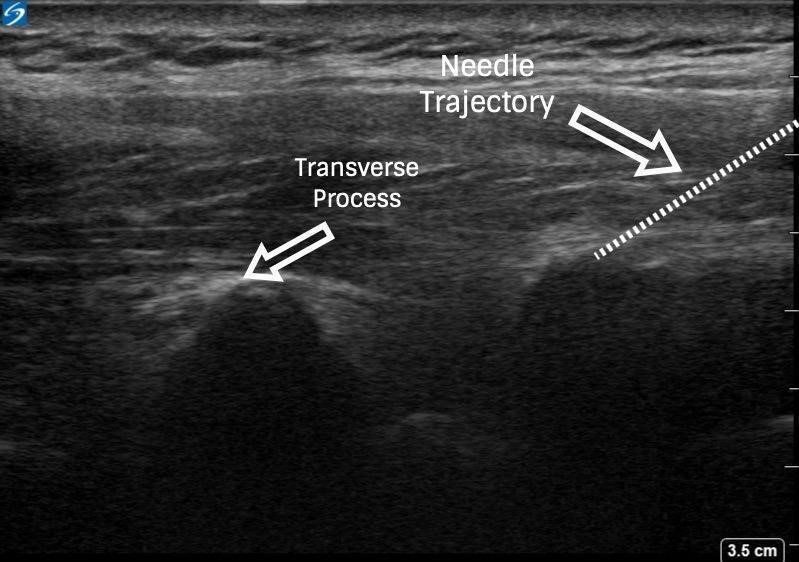
With PVB, deposit local anesthetic between the pleural and costotransverse ligament under ultrasound guidance using either an in-plane or out-of-plane needle approach. For the out-of-plane technique, hold the transducer (linear or curvilinear, depending on the patient’s body habitus) in the sagittal plane, allowing for a linear needle trajectory perpendicular to the skin (Figure 3). The pleura is visible in that plane between the ribs laterally and the transverse processes medially. In the transition from rib to transverse process, the bone image appears larger and squarer as the transducer is moved more medially (Figure 4). Hydrodissect with sterile saline to estimate the needle tip location when using an out-of-plane technique, with the downward/anterior bowing of pleura, or loss of reflection of saline upward/posterior, indicating injection into the paravertebral space. This is the preferred regional anesthetic technique at our institution and has provided effective surgical anesthesia for a variety of oncologic breast and thoracic procedures.
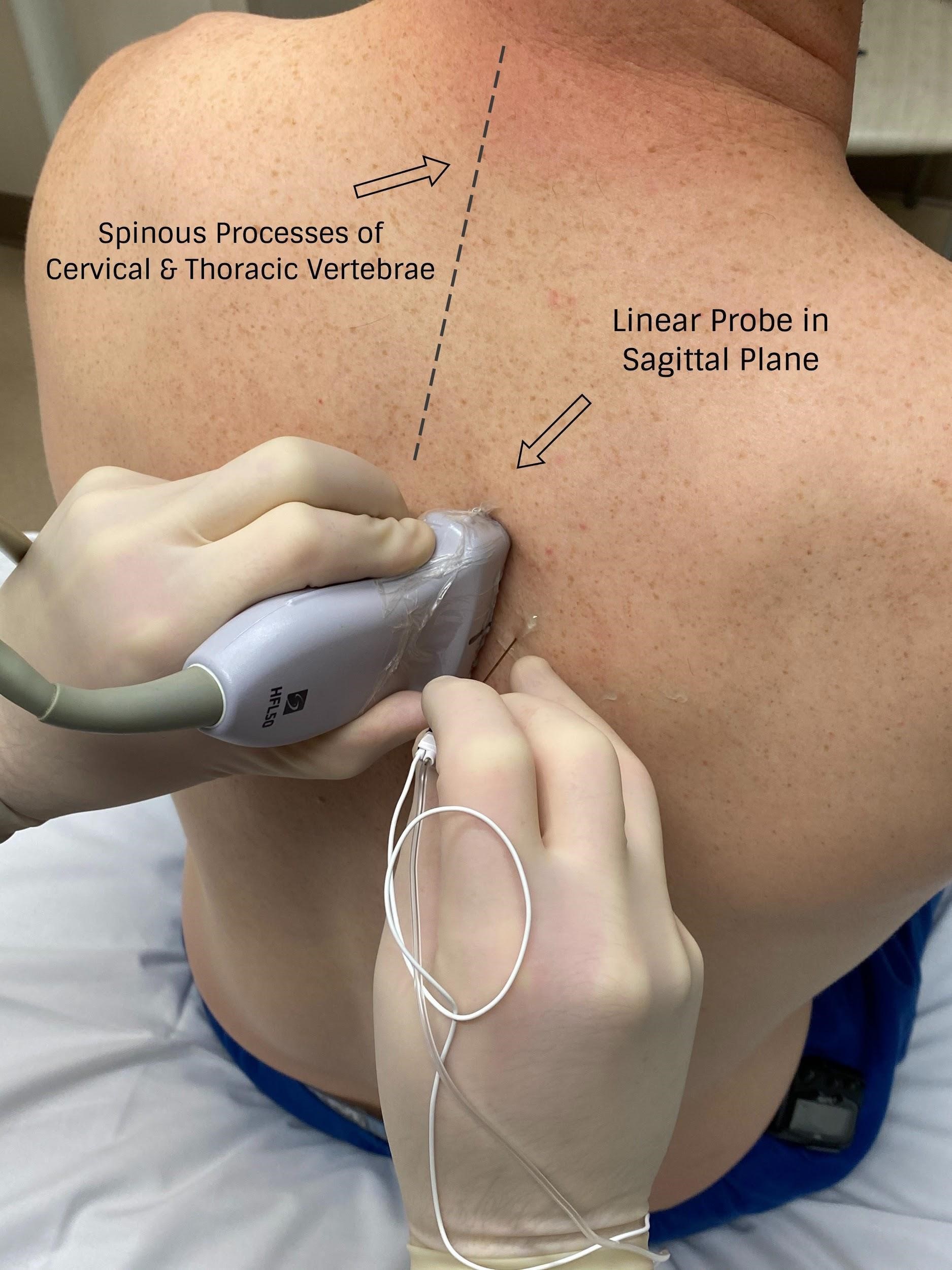
Figure 3: Probe position for out-of-plane paravertebral block
Image courtesy of Steven Ethier, DO.
Local anesthetic may be deposited at a single site or at multiple spinal nerve levels. A single injection of high-volume (15–20 mL 0.5% bupivacaine) can reliably produce a somatic block over five dermatomes and a sympathetic block over eight dermatomes.3 Current evidence also demonstrates that a single injection of 15–20 mL 0.375%–0.5% bupivacaine is as effective as multiple injections of 3–4 mL per site in covering approximately five dermatomes.3
To facilitate surgical anesthesia, use of low-volume PVBs at a number of spinal nerve levels may improve efficacy. This can be done by skipping one to two segments in between injections and using a decreased volume (approximately 8–12 mL) of local anesthetic. This will not only ensure coverage of more than five dermatomes but also minimize spread into the epidural space and decrease the risk of a bilateral sensory blockade.3 Determination of PVB injection sites should be guided by knowledge of the planned surgical site (eg, sentinel lymph node biopsy, axillary involvement, upper-versus lower-quadrant lesions) (Figures 3 and 4 and Table 3).
PVB’s advantages for breast surgery include improved postoperative pain scores, reduced analgesic consumption, and potential avoidance of general anesthesia.[1] Evidence also indicates that the incidence and severity of chronic pain may be reduced following breast surgery performed under PVB.[1] In addition, physical and mental well-being may be significantly improved with the use of paravertebral analgesia.[4] Initial retrospective studies created excitement for its use by demonstrating a decreased breast cancer recurrence rate with PVB versus opioid-based analgesic regimens. Unfortunately, a recently published multicenter trial failed to demonstrate a significant cancer recurrence benefit associated with PVB.[5] When compared with epidural anesthesia, PVBs are associated with a decreased incidence of hypotension, urinary retention, respiratory issues, and postoperative nausea and vomiting.[3]
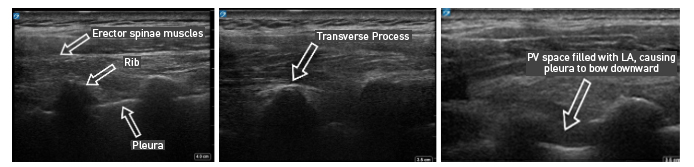
Figure 4: Ultrasound images of paravertebral block via out-of-plane technique
Risk factors specific to PVB include pleural puncture (1.1%) or pneumothorax (0.5%), inadvertent epidural or spinal injection, transient ipsilateral Horner’s syndrome (2%), and sensory changes to the arm.[3] The block may also be associated with a high degree of systemic local anesthetic absorption, and patients should be monitored closely for the development of local anesthetic systemic toxicity. In our extensive clinical experience, PVBs also appear to be associated with greater patient discomfort compared to other blocks (ie, PECS II). Pleural puncture is rare, usually associated with minor clinical significance, and can generally be managed conservatively with observation and a high index of suspicion for the development of pneumothorax (0.5% incidence).[3] Epidural injection may be more common with larger volumes (> 25 mL) of injectate, and intrathecal and intravascular injections can be minimized by aspirating prior to local anesthetic injection.[3] Horner’s syndrome (ie, oculosympathetic palsy) can result from sympathetic blockade traversing cephalad to the stellate ganglion or to sympathetic preganglionic fibers. If it occurs bilaterally, significant epidural spread should be considered, and patients should be monitored closely for the development of hypotension, respiratory depression, and motor weakness. Lastly, sensory changes in the arm can occur from a superior PVB (ie, T1) and local anesthetic spread to C8 and T1.[3]
Table 3: Summary of chest wall innervation, breast surgeries, and analgesic procedures.[2] (Click to enlarge image.)
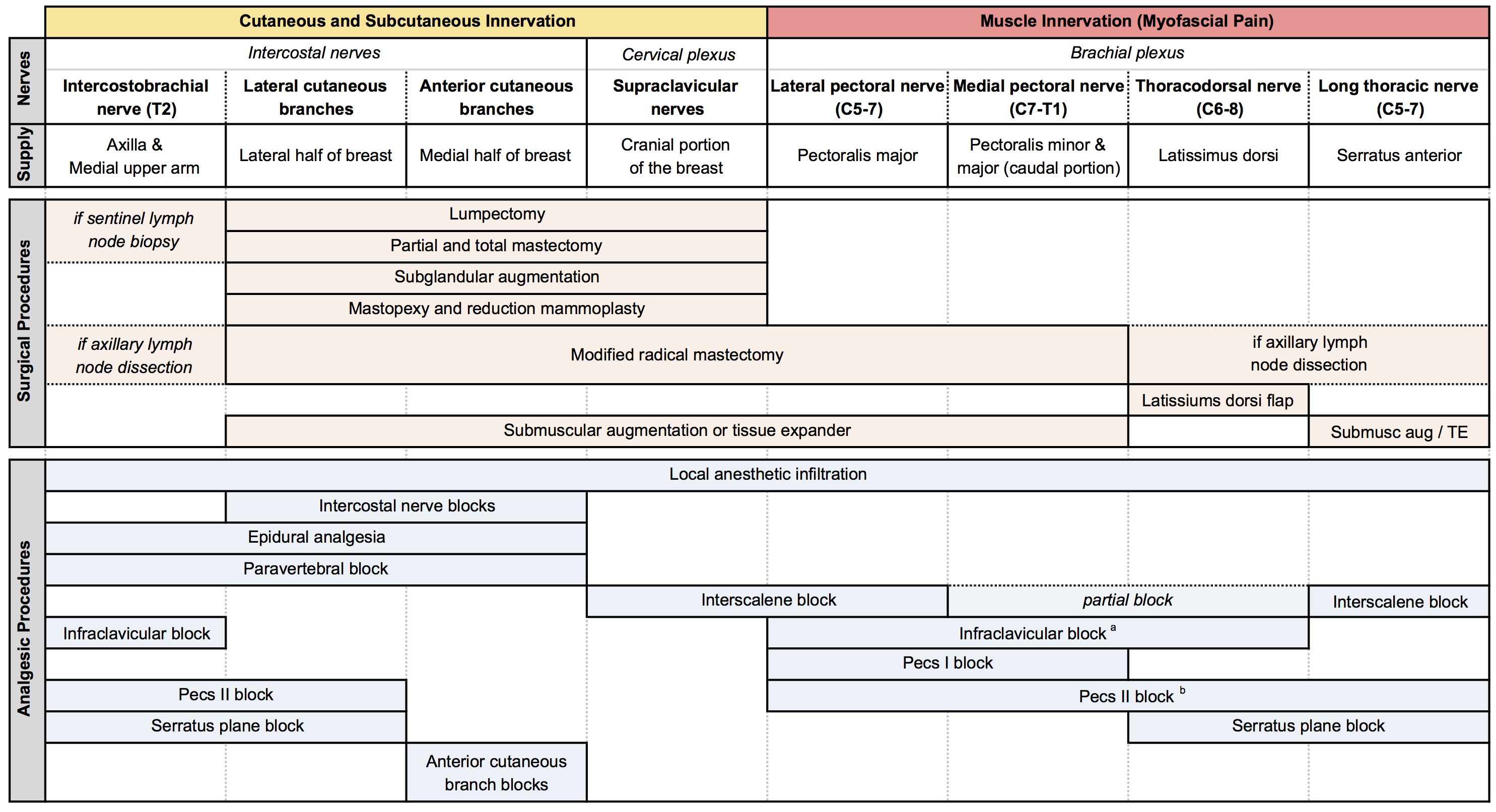
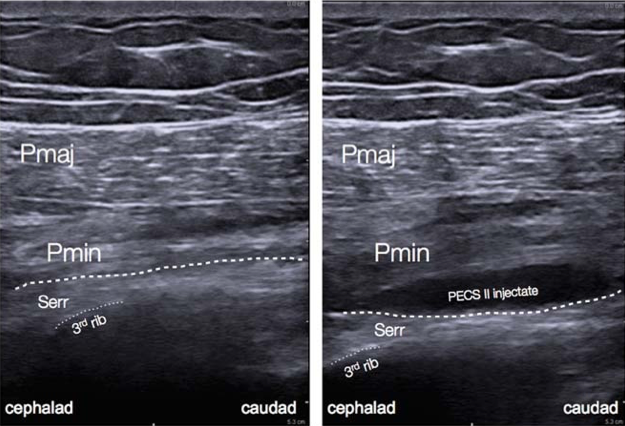
Figure 5: Ultrasound anatomy of pectoral nerve block II.[12
Pectoral Nerve Block
PECS blocks are becoming an increasingly popular technique for the provision of breast analgesia. PECS I blocks the medial and lateral pectoral nerves, which are situated in between the pectoralis minor and major muscles on the anterolateral chest. PECS II includes the PECS I block and an injection of local anesthetic into a plane more lateral on the chest wall and between the pectoralis minor and serratus anterior muscles, targeting the lateral cutaneous branches of the intercostal nerves, long thoracic nerve, and thoracodorsal nerve (Figure 5).[2] PECS II has been shown to be associated with lower postoperative pain scores and opioid consumption similar to PVB. However, the data is currently limited in terms of confirming which block is superior and they can both provide surgical anesthesia. The PECS block may be better tolerated by patients and easier to perform in the setting of decreased ancillary support or in an anesthetized patient. More extensive surgeries may require additional blocks to provide coverage for axillary procedures or medial breast analgesia.[6]
Figure 6: Transverse approach to erector spinae plane block
Erector Spinae Plane Block
Since the ESPB was first described, the number of clinical scenarios that might benefit from it has increased exponentially. The ESPB consists of an injection of local anesthetic between the erector spinae muscles and transverse processes of the desired vertebrae. The mechanism of analgesic effect for the ESPB is somewhat unclear and may be related to either anterior diffusion to spinal nerve roots or lateral spread to intercostal nerves.[7] Similar to PVB, ESPBs can be performed with needle insertion in or out of plane relative to the ultrasound probe and with single or multiple injections (Figure 6). With an ESPB, the needle target is posterior to the transverse process itself, and the presence of this bony impediment should minimize the risk of inadvertent pleural puncture. To provide maximal analgesic efficacy, local anesthetic is visualized layering beneath the erector spinae muscle.
Although ESPB’s ability to provide surgical anesthesia is currently not well understood, multiple published case reports have described its successful use as the primary anesthetic for breast surgery. One of ESPB’s potential advantages is its ability to improve postoperative pain over multiple dermatomes with a single injection. ESPBs may also be technically easier to perform and less likely to result in uncontrolled bleeding that leads to significant nerve or lung injury. However, few studies have proved or disproved those theoretical benefits. ESPBs are also closely associated with highly vascularized muscle planes, and a few cases of local anesthetic systemic toxicity have been reported.[7]
Serratus Plane Block
The serratus plane block is another useful adjunct for chest surgery and is performed more lateral and caudad than the PECS II at the fifth rib in the midaxillary line. Local anesthetic is deposited between the serratus anterior and the latissimus dorsi muscles, with the thoracodorsal artery being an indicator of the plane’s location, to provide a sensory block to the T2–T9 dermatomes via extended intercostal nerve coverage. Local anesthetic can also be deposited in the plane deep to the serratus anterior muscle with similar analgesic efficacy. A PECS I block is required to provide analgesia to pectoral muscles, and, similar to a PECS II block, medial breast incisions will require local anesthetic supplementation.[2]
How I Do It: PVB for Breast Surgery
In our clinical practice, a thoracic PVB is generally performed with ultrasound guidance with the probe oriented in the sagittal plane and the needle inserted out of plane relative to the probe. To provide surgical anesthesia, PVBs are generally performed at T2, T4, and T6. Essential equipment includes the ultrasound machine (linear probe of 10–13 MHz), local anesthetic, chlorhexidine or iodine prep solutions, block needle (typically 5 cm, rarely 10 cm), three-way stopcock, multiple sterile saline flushes, transparent dressing (or ultrasound probe cover for bilateral surgery), ultrasound gel, sterile gloves, additional faculty to assist with positioning and local anesthetic injection, and sedative and rescue medications.
With the patient seated, apply standard ASA monitors, administer supplemental oxygen, and initiate intravenous fluid administration. Sedation generally includes midazolam (2 mg) and/or fentanyl (50–100 mcg). Following sterile prep, position the ultrasound probe in the sagittal plane adjacent to the planned vertebral level approximately 3 cm off of the midline of the spine (Figure 3). Scanning from medial to lateral, first identify the transverse process and rib, taking note of their transition by change in shape and depth (Figure 4). Both structures will be seen in cross-section with pleura located anterior to the cephalad and caudad bony structures. Targeted injections should occur after the transition from rib to transverse process (the transverse process is located more medially and appears more squared-off in appearance).
Taking note of the depth of the pleura, center the structure on the ultrasound screen and anesthetize the patient’s skin with lidocaine lateral and adjacent to the middle of the probe. The costotransverse ligament is located posterior to the pleura (occasionally difficult to visualize on ultrasound imaging) and injections in the space between the costotransverse ligament and pleura should provide effective PVB. Insert the block needle approximately 1–2 cm less before the pleura using the depth indicators on the nerve block needle. The local anesthetic for the block and a syringe of sterile saline are attached to the block needle via a three-way stopcock, initially open to saline and previously flushed with saline to remove all air. Give a small but firm push of the saline flush to provide an estimation of needle tip position. Advance the needle in small increments until the saline is no longer seen reflecting superficially from the pleura. Placing the needle tip in the paravertebral space will result in anterior pleural deflection in a bowing-like manner and can usually be visualized spreading superiorly and inferiorly in adjacent paravertebral spaces. Skip one or two levels between injection points, depending on the incision site (eg, SLNB in axilla), tumor location, or patient anatomy.
Once the block is completed, place the patient supine. The onset time is approximately 15 minutes, and block duration is generally 12–18 hours.
Intraoperative management with a planned surgical PVB typically consists of sedation with a propofol infusion and supplementation with 50–100 mcg fentanyl or 10–25 mg intermittent ketamine boluses. Be vigilant regarding local anesthetic systemic toxicity. Given the large volume of local anesthetic and the long-acting nature of bupivacaine, a maximum dose of local anesthetic on all patients is based on ideal body weight. It should be noted that the axilla is not as reliably covered with a PVB. Therefore, calculate the remaining anesthetic available for the surgeon to use intraoperatively, should supplementation be required. Communicate the dose of available local anesthetic to the intraoperative staff to discuss during their surgical preoperative timeout.
RA Versus General Anesthesia for Breast Surgery and Cancer Recurrence
The ability of anesthetic technique (ie, RA or general anesthesia) to affect cancer recurrence rates following curative oncologic surgery has received significant academic attention. Theory suggests that RA may reduce recurrence rates by reducing the perioperative requirement for analgesics that diminish immune system function. However, no current high-quality data confirms that theory.
In 2019, The Lancet published a multicenter study of patients undergoing breast cancer resection who were randomized to receive either PVB and propofol infusion or general anesthesia with sevoflurane and opioids. Primary outcome measurements included local and metastatic recurrence, whereas secondary outcomes included incisional pain at 6 and 12 months. The researchers found that RA did not reduce recurrence rates compared to general anesthesia, nor did it affect the incidence of persistent incisional or neuropathic breast pain.[5]
Nonetheless, growing numbers of laboratory and animal studies correlate the choice of anesthetic drug with activity of cancer cells. However, high-quality, randomized, controlled clinical trials are few and far between. Thus, although anesthetic agents may affect cancer cells, there remains only limited data to suggest a change in practice with regard to preventing recurrence for patients undergoing oncologic surgery.[8–11]
References
- Sherwin A, Buggy DJ. Anaesthesia for breast surgery. BJA Educ. 2018;18(11):342–348. https://doi.org/10.1016/j.bjae.2018.08.002
- Woodworth G, Ivie RMJ, Nelson SM, Walker CM, Maniker RB. Perioperative breast analgesia: a qualitative review of anatomy and regional techniques. Reg Anesth Pain Med. 2017;42(5):609–631. https://doi.org/10.1097/AAP.0000000000000641
- Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95(3):771–780. https://doi.org/10.1097/00000542-200109000- 00033
- Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014;39:289- 298. https://doi.org/10.1097/AAP.0000000000000113
- Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394(10211):1807–1815. https://doi.org/10.1016/S0140-6736(19)32313-X
- Parras T, Blanco R. PECS blocks. Available at https://www.wfsahq.org/components/com_virtual_library/media/7ef4612aa547d759a2e24087424e9bba-ATOTW346.pdf. Published January 31, 2017. Accessed February 23, 2020.
- Pourkashanian A, Narayanan M, Venkataraju A. The erector spinae plane block: a review of current evidence. https://resources.wfsahq.org/atotw/the-erector-spinae-plane-block-a-review-of-current-evidence-2/. Published December 3, 2019. Accessed February 23, 2020.
- Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i56–i62. https://doi.org/10.1093/bja/aeu200
- Buggy DJ, Wall T. Can anaesthetic-analgesic technique during cancer surgery of curative intent influence recurrence or metastasis? An update. Br J Anaesth. 2019;123(6):e525–e526. https://doi.org/10.1016/j.bja.2019.07.023
- Desmond F, McCormack J, Mulligan N, et al. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35(3):1311–1319. http://ar.iiarjournals.org/content/35/3/1311.full
- Garg, R. Regional anaesthesia in breast cancer: benefits beyond pain. Indian J Anaesth. 2017;61(5):369–372. https://doi.org/10.4103/ija.IJA_292_17
- Yalamuri S, Klinger RY, Bullock WM, et al. Pectoral fascial (PECS) I and II blocks as rescue analgesia in a patient undergoing minimally invasive cardiac surgery. Reg Anesth Pain Med. 2017;42(6):764–766. https://doi.org/10.1097/AAP.0000000000000661
Leave a commentOrder by
Newest on top Oldest on top