2016 ASRA Chronic Pain Grant Update
Effectiveness of OnabotulinumtoxinA (Botox®) in Pediatric Patients Experiencing Migraines: A Randomized Double Blinded Placebo Crossover Study in the Pediatric Pain Population
Principal Investigator: Shalini Shah, MD
Faculty Mentor: Joseph Rinehart MD
Department of Anesthesiology & Perioperative Care
University of California, Irvine
While many chronic pain conditions are manageable, migraine pain can be devastating. Migraine episodes are unpredictable in onset and duration and profoundly debilitating for sufferers. Recently, onabotulinumtoxinA (OBTA) was approved for the prophylaxis of adult migraine symptoms by the U.S. Food and Drug Administration (FDA), which has dramatically altered the way pain physicians approach migraine pain.
Most adults who suffer with migraines have their first headache during childhood or adolescence.[1] Although it appears that many preventative agents are safe in children, none are currently FDA-approved for this age group, apart from topiramate which achieved “on-label” status as of 2014. As a result, despite experiencing significant disability, the vast majority of children who present to their physician with migraine headache do not receive prophylactic therapy.[2] A study published in JAMA in 2003 found that health care costs, work-related disability for parents, and lost educational opportunity for the child leads to an annual economic impact in the U.S. of approximately $36 billion due to both direct medical costs and lost productivity into adulthood.[3]
The significance of this study is to evaluate the efficacy of OBTA (sold commercially as “BOTOX®”) for the treatment and prophylaxis of pediatric migraine in a randomized, double-blinded, placebo crossover study. Our hypothesis is that OBTA would have a statistically significant decrease in the three endpoints of migraine symptomology: intensity, frequency, and duration of migraine. No trials currently exist in literature studying OBTA for efficacy and/or safety for indication of pediatric migraine, although significant contributions have been made by retrospective case series over the last 10 years.[2,7,8,9,10] Additional historical and longitudinal interest for the design of this study comes from the principal investigator’s (PI) extensive use of “off label” OBTA to treat refractory pediatric migraine over the last five years.
Looking at our historical off-label data, we realized we had an ideal candidate to investigate for ASRA’s goal of identification of novel applications of existing therapeutics. Moreover, this trial may benefit an under-studied pain population with the long-term aim of obtaining a new FDA indication to “on label” status. The proposal also carries policy and legislative impact as it fulfills the federal initiative to design and conduct trials in the pediatric pain population within confines of the Best Pharmaceuticals for Children Act (BPCA) of 2002. The goal of the BPCA Program is to improve pediatric therapeutics through preclinical and clinical drug trials that lead to drug labeling changes.
The study itself has three specific aims:
Aim 1: To test if onabotulinumtoxinA is superior to placebo in reducing headache frequency, intensity and pediatric migraine-related disability (efficacy)
Aim 2: To evaluate the incidence of adverse events of onabotulinumtoxinA administration in children aged 8-17 (safety, tolerability)
Aim 3: To evaluate if onabotulinumtoxinA can contribute to reduction in preventive and rescue medication and reduction in health care utilization via emergency room and hospital admissions and health care costs (hospital and pharmacy resource utilization)
The study aims fit together in an overall framework such that primary and secondary outcomes are easily defined, measureable, and validated as an acceptable means to measure clinical success and to longitudinally assess response.
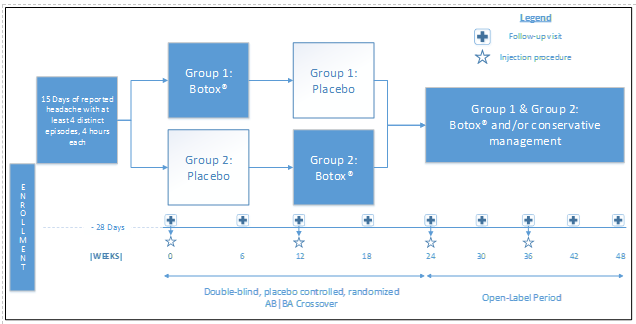
Study Design
An AB|BA crossover design (which uses each patient has his/her own control, both minimizing recruitment numbers and the need for a control group which never receives treatment with OBTA) was selected as the best option after consideration of the alternatives. The influence of confounding covariates is reduced because each crossover patient serves as his or her own control. A four-week baseline prior to treatment will act as a no-treatment control to compare to both the treatment and placebo.?The study does not exclude patients on preventive or abortive migraine medication, in an attempt to demonstrate superiority on study drug over conservative medical management. Following the crossover period, all patients will then proceed to an open-label treatment phase with OBTA.
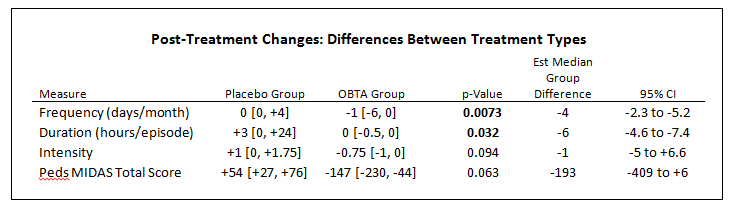
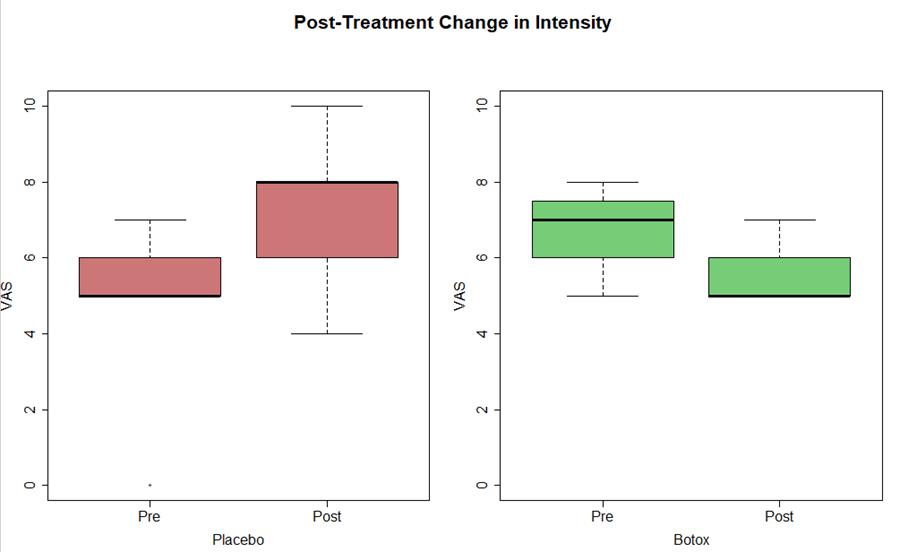
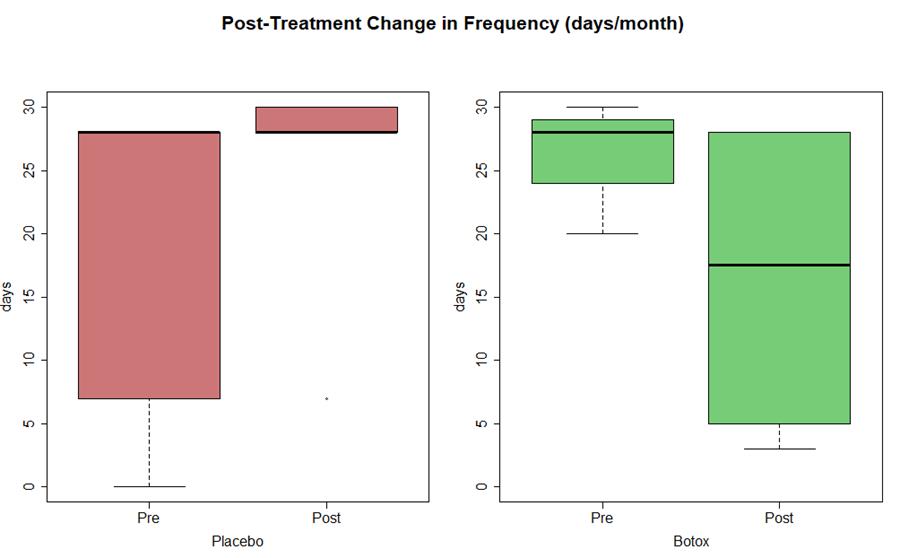
Preliminary Data Results and Conclusion:
- Preliminary data 6 subjects
- Statistically significant (P <0.05) decrease in frequency (est median -4 days) and duration of migraine (est median -6 hours)
- Trending decrease in intensity of migraine (est median -1 NRS), not yet statistical significant change (p=0.09)
- Disability scoring (Pedi MIDAS) trend towards functional improvement with OBTA but not placebo (P = 0.06)
- Encouraged
- Thank you to ASRA and mentor Joseph Rinehart, MD.
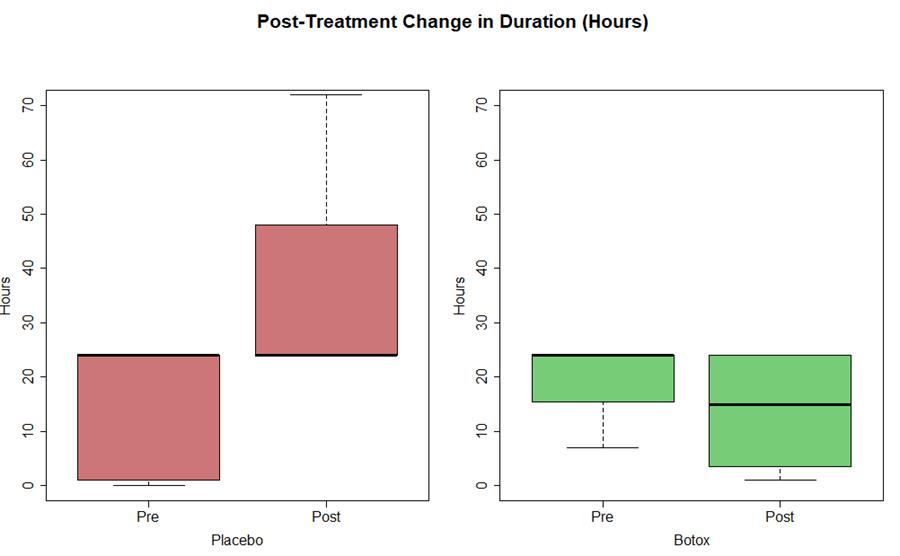
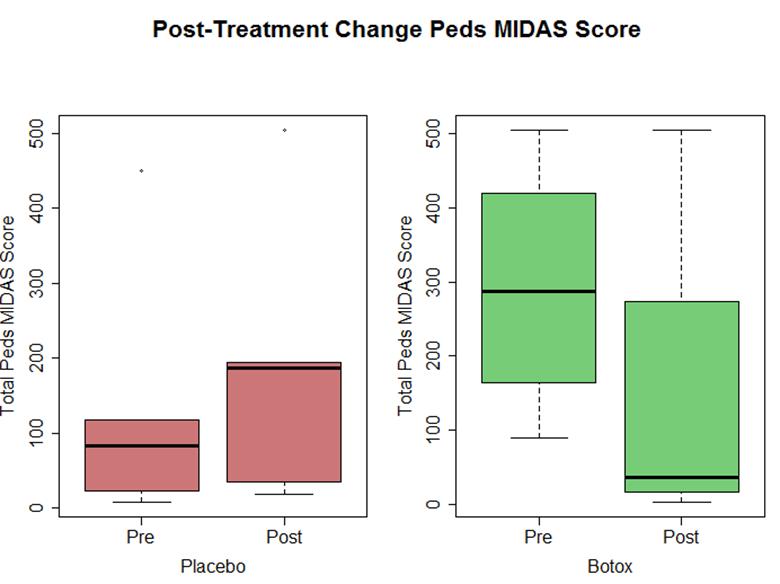
References
- ^ Jacobs, H. and J. Gladstein, Pediatric headache: a clinical review. Headache, 2012. 52(2): p. 333-9.
- ^ Hershey, A.D., et al., Childhood and Adolescent Migraine Prevention (CHAMP) study: a double-blinded, placebo-controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in the prevention of childhood and adolescent migraine. Headache, 2013. 53(5): p. 799-816.
- ^ Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003; 290:2443-2454.
- Delgado MR, Hertz D, Aisne M, et al. Quality Standards Sub-committee of American Academy of Neuology and the Practice Committee of the Child Neurology Society. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidenced-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2010;74:336-43.
- Aurora, S.K., et al., OnabotulinumtoxinAfor treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache, 2011. 51(9): p. 1358-73.
- Dodick, D.W., et al., OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache, 2010. 50(6): p. 921-36.
- Ahmed K, Oas KH,, Mack KJ, Garza I. Experience with botulinumtoxin type A in medically intractable pediatric chronic daily headache. Pediatr Neurol.2010;43:316-19.
- Chan, V.W., E.J. McCabe, and D.L. MacGregor, Botox treatment for migraine and chronic daily headache in adolescents. J Neurosci Nurs, 2009. 41(5): p. 235-43.
- Kabbouche, M., H. O'Brien, and A.D. Hershey, OnabotulinumtoxinA in pediatric chronic daily headache. Curr Neurol Neurosci Rep, 2012. 12(2): p. 114-7.
- Schroeder, A.S., et al., Ten-year follow-up in a case series of integrative botulinum toxin intervention in adolescents with chronic daily headache and associated muscle pain. Neuropediatrics, 2012. 43(6): p. 339-45.
Leave a commentOrder by
Newest on top Oldest on top