Gastric Ultrasonography for the Regional Anesthesiologist
This article originally appeared in ASRA News, Volume 17, Number 1, pp. 14-16 (February 2017).
Authors
Susan Bragg, MD Resident
Department of Anesthesia
Richelle Kruisselbrink, MD, FRCPC Staff Anesthesiologist
Toronto Western Hospital–University Health Network
Lecturer of Anesthesiology
Anahi Perlas, MD, FRCPC
Staff Anesthesiologist, Toronto Western Hospital–University Health Network
Associate Professor of Anesthesiology
University of Toronto
Toronto, Ontario, Canada
Pulmonary aspiration of gastric contents is a serious perioperative complication. Mortality after aspiration pneumonia can be as high as 5%, and it is the leading cause of airway-related deaths in anesthesia.[1,2] An important risk factor
for aspiration is the presence of gastric contents at the time of induction. We rely on recommended fasting intervals to minimize aspiration risk; however, current guidelines were developed for healthy patients undergoing elective procedures and may not be applicable to urgent surgical cases or to patients with comorbidities that delay gastric emptying. Gastric ultrasound is a convenient bedside tool to directly assess a surgical patient’s gastric contents that can be learned and performed quickly and has been shown to affect anesthetic management.[3,4]
In this article, we will discuss how gastric ultrasound can be a useful tool for the regional anesthetist to minimize the risk of perioperative aspiration.
Clinical Scenario
A 32-year-old woman presents for an urgent debridement and open reduction internal fixation of an open distal radial fracture. Her medical history is significant for severe asthma, with a recent exacerbation. You plan to perform an axillary block; however, she is very anxious, and you determine that she will likely require sedation. On further assessment, she states that she ate a yogurt and a piece of toast 4 hours ago. In this scenario, the use of gastric ultrasonography can help assess her fasting status and direct clinical management.
Basics of Gastric Ultrasound
The goal of point-of-care gastric ultrasound is to evaluate the gastric antrum, as the content and size of the antrum correlate with the content and volume of the entire organ. Scanning with a curvilinear probe in a sagittal plane over the epigastrum, the antrum appears round or oval and is found between the liver anteriorly and the pancreas posteriorly. The aorta or inferior vena cava is located posterior to the pancreas. The superior mesenteric artery can sometimes also be seen (Figure 1). The antrum has a characteristic five-layered wall. These five layers alternate between hyper- and hypoechoic. Although the antrum itself is located at an average depth of 4–5 cm, an initial scanning depth of 12–15 cm is recommended to properly identify all regional anatomical landmarks.
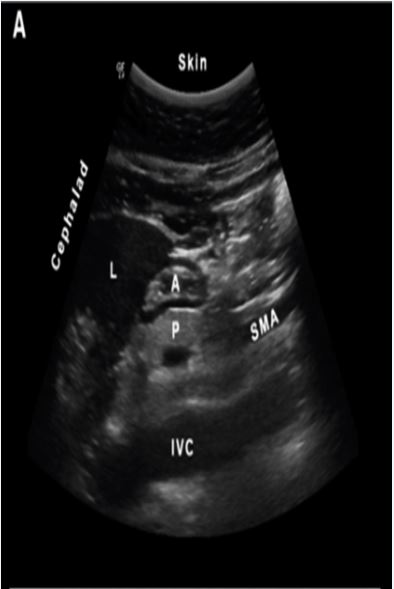
Figure 1: Sagittal scan of an empty antrum. A = gastric antrum; L = liver; P = pancreas; SMA = superior mesenteric artery; IVC = inferior vena cava. Reproduced with permission from Perlas et al.[5]
Tip: The gastric antrum has five distinct layers, which helps differentiate it from loops of small bowel and the colon.
The patient is examined in both the supine and the right lateral decubitus (RLD) positions. In the RLD position, a greater proportion of the fluid and solid contents move with gravity toward the more dependent antrum, making it a more sensitive position to evaluate gastric content. At the same time, the air content moves away from the antrum toward the more proximal body or fundus, thus further facilitating the examination.
Tip: Applying gentle pressure and examining at the end of deep inspiration may help bring the structures into view.
Interpreting the Views
An empty antrum will have contracted, thickened walls, giving it a target or bull’s-eye appearance (Figure 2A). The anterior and posterior walls are juxtaposed against each other. A clear fluid-filled antrum will be distended, with thin walls and homogeneous, hypoechoic content. Air and gas bubbles may be present and appear as mobile punctate echoes, giving the antral lumen a “starry night” appearance (Figure 2B).
Solid contents are heterogeneous with a mixed echogenicity (Figure 2D). Air is often entrained with a food bolus during chewing and swallowing. The air-solid mixture creates artifacts that may obscure the posterior wall. This is called the frosted-glass sign (Figure 2C) and is associated with a recent solid meal.
Tip: An empty antrum can be the hardest to identify. Use the anatomical landmarks to guide you.
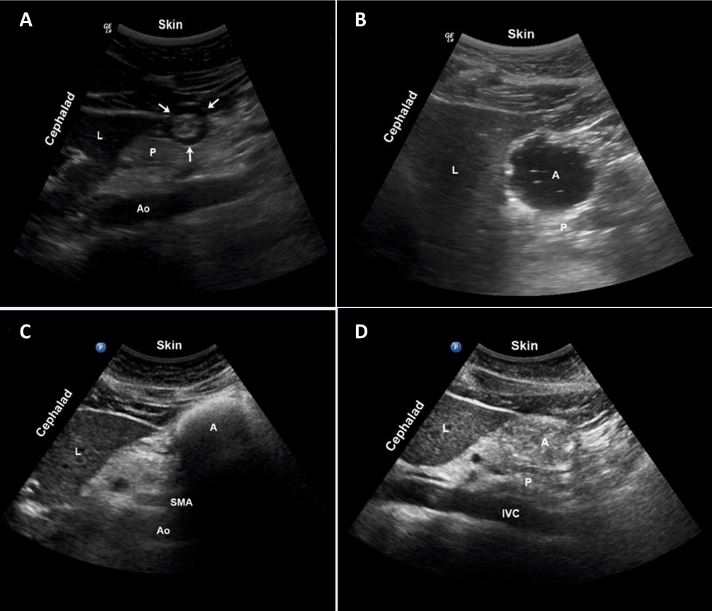
Figure 2: (A) Empty antrum, "bull's eye." (B) Clear fluid with air bubbles, "starry night." (C) Solid contents with air, "frosted glass." (D) Solid contents. Adapted and reproduced with permission from Cubillos et al.[6]
Qualitative Aspiration Risk Assessment
Translating these views into an assessment of aspiration risk can be done with a grading system, as shown in Table 1.
A grade 0 antrum appears empty in both positions and indicates no gastric content. A grade 1 antrum is empty when supine but demonstrates clear fluid in RLD. This is a common finding in healthy fasted patients (about 50% of the time). Previous studies have shown that a grade 1 antrum correlates with a low volume of gastric fluid volume, consistent with baseline gastric secretions (up to 1.5 mL/kg or about 100 mL in a 70-kg adult).[5] Both grade 0 and grade 1 are common findings in fasted healthy subjects and are therefore considered markers of “low risk.”
A grade 2 antrum, on the other hand, contains clear fluid in both supine and RLD positions. A grade 2 antrum is an uncommon finding in fasted patients (3–5% of cases) and usually correlates with higher than baseline volume, therefore being a possible marker of a higher aspiration risk.
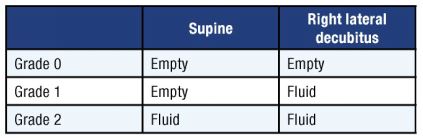
Table 1: Gastric content grading system to determine aspiration risk.
Finally, any amount of solid food in the stomach is inconsistent with a fasted state and should be considered a “full stomach” with higher-than-baseline aspiration risk.
Quantitative Assessment: How Much Is Too Much?
The grading system is a quick screening test and provides a conservative approach for patients with clear fluid content. However, a quantitative evaluation can also be done to more accurately determine the gastric volume. A cross-sectional area of the gastric antrum (in cm2) is measured in the RLD position, at the level of the abdominal aorta, using a free-tracing method and the ultrasound machine caliper. The measurement should include the outermost serosal layer of the antrum.
A precise gastric volume can be estimated based on the antral cross-sectional area (CSA) and the patient’s age (Table 2):
Volume (mL): 27.0 + 14.6 ´ Right Lateral CSA – 1.28 ´ Age (years)5
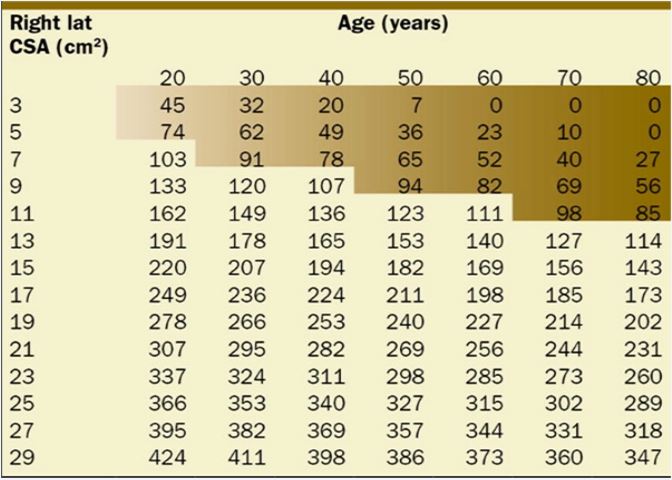
Table 2: Predicted gastric volume (mL) based on gastric antral cross-sectional area (cm2), stratified by patient age.
A volume of <1.5 mL/kg is within the normal range for a healthy fasted individual and therefore carries a low risk of aspiration. Volumes in excess of 1.5 mL/kg are quite rare in healthy fasted individuals and could therefore pose a higher-than-baseline aspiration risk.
Clinical Scenario Revisited
You perform a gastric ultrasound examination that shows an empty antrum when supine but clear fluid in the RLD position. This suggest there is no solid food content in the stomach any longer and that the volume of clear fluid remaining is likely less than 100 mL (a grade 1 antrum). For a more precise evaluation, you use the free tracing method to measure antral CSA, and you calculate her gastric volume to be 80 mL. This level of gastric volume is within the normal range of values for a fasted healthy adult of her weight (75 kg), therefore suggesting a baseline or low aspiration risk. Based on this information, you proceed with an axillary block with sedation, and she has an uncomplicated perioperative course.
References
- Shime N, Ono A, Chihara E, Tanaka Y. Current status of pulmonary aspiration associated with general anesthesia: a nationwide survey in Japan. Masui. 2005;54:1177–1185.
- Cook T, Frerk C. 4th National Audit Project (NAP 4): major complications of airway management in the United Kingdom report and findings—chapter 19. Aspiration of gastric contents and of blood. 2011. March The Royal College of Anaesthetists and The Difficult Airway Society. Br J Anaesth. 2016;117(2):182–
190. doi: 10.1093/bja/aew177. - Arzola C, Carvalho JC, Cubillos J, Ye XY, Perlas A. Anesthesiologists’ learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anesth. 2013;60:771–779. doi:10.1007/s12630-013-9974-y.
- Alakkas H, Kruisselbrink R, Chin KJ, et al. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anesth. 2015;62(11):1188–1195.
- Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–363.
- Cubillos J, Tse C, Chan VW, Perlas A. Bedside ultrasound assessment of gastric content: an observational study. Can J Anesth. 2012;59(4):416–423.
Leave a commentOrder by
Newest on top Oldest on top