Myofascial Pain and Fibromyalgia Syndrome
Authors
Alik Saidov, MD
Resident Physician
Henry Kroll, MD
Clinical Associate Professor
Program Director, Pain Medicine Fellowship
Department of Anesthesiology
Wayne State School of Medicine
Henry Ford Hospital
Detroit, MI
Myofascial Pain Syndrome
Introduction
Myofascial Pain Syndrome (MPS) is a regional pain syndrome originating from soft tissue with manifestation of sensory, motor, and autonomic symptoms caused by myofascial trigger points (MTrP).[1-3] MTrP is best defined as a hyperirritable spot in the skeletal muscle that is associated with a hypersensitive palpable nodule in a taut band. The spot is painful on compression and can give rise to a characteristic referred pain and tenderness pattern (Figures 1, 2).[2][4]
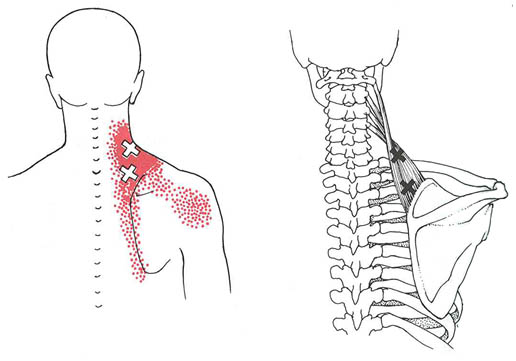
Figure 1. Consolidated referred pain pattern for trigger point regions of the right levator scapulae muscle. The essential pain pattern is solid red, and the spillover pattern is stippled red. The upper X locates MTrP in mid portion of the muscle that is often overlooked. The lower X locates the much more obvious trigger area tenderness commonly found near the region of the muscle’s scapular attachment, which often is enthesopathy secondary to taut band tension associated with the MTrPs. Adapted from Simons DG, Travell JG, Simons LS: Myofascial Pain and Dysfunction: The Trigger Point Manual, vol 1, ed 2. Baltimore: Williams & Wilkins; 1999.
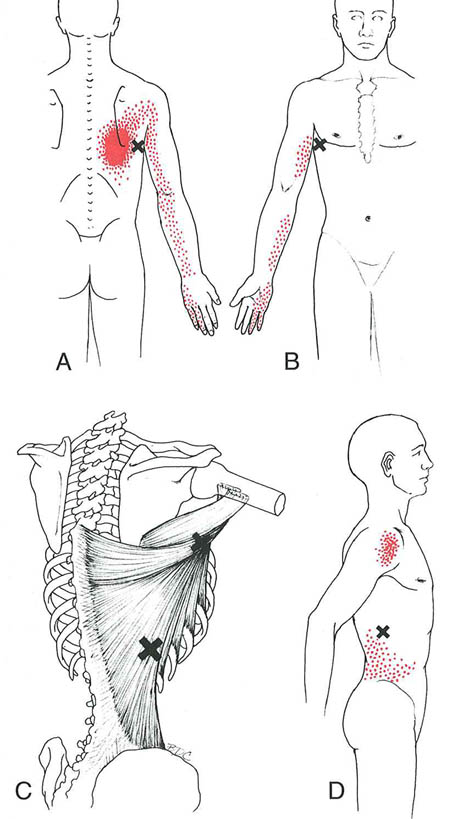
Figure 2. Referred pain patterns (essential portion is solid red, spillover portion is stippled red) referred from trigger points (Xs) in the right latissimus dorsi muscle. A, back view of the pain pattern from trigger points in their most common location within the axillary portion of the muscle. B, front view of same. C, anatomical view: most common, superior, location of trigger points (upper X) and inferior location (lower X). D, pain pattern of the inferior trigger point, which may also refer pain down the arm.
MPS is extremely common. Bennet has reported that 44 million individuals in the United States, alone, have an active MTrP. Some reports indicate the presence of latent MTrPs in 45% of asymptomatic male and 54% female subjects.[5] While active MTrPs will be most commonly found in vigorous individuals between the ages of 31 to 50, the elderly population with a reduced level of activity, stiffness, and restricted range of motion, still harbor latent MTrPs.[6] Not surprisingly, the economic burden of industrial lost time and medical compensation is substantial.[7]
Diagnostic Criteria
No laboratory test or imaging technique has been established as diagnostic of MPS. The diagnosis is dependent upon eliciting a detailed history, exhaustive physical examination with manual palpation of soft tissue, and clinical judgment. Manual palpation, however, is considered to be subjective and unreliable[8] and special training in palpation techniques is usually required to obtain a common agreement in the judgment of palpation criteria.[9-10] Some muscles are particularly difficult to evaluate manually because of their deep anatomical locations. Simons and Travell have proposed criteria for the diagnosis of MTrPs, which include Essential Criteria and Confirmatory Observations (Table 1).[2]
| Criterion | Description |
|---|---|
| Adapted from Simons DG, Travell JG, Simons LS: Myofascial Pain and Dysfunction: The Trigger Point Manual, vol 1, edn 2. Baltimore: Williams & Wilkins; 1999. | |
| Essential Criteria |
|
| Confirmatory Observations |
|
Manipulation by snapping palpation or penetration with a needle of a MTrP’s taut band usually induces a local twitch response (LTR). The LTR is due to the transient reflex contraction of a group of tense muscle fibers that traverse a trigger point. This LTR has also been referred to as a "jump sign."[2] Deep palpation over a currently tender soft tissue area that leads to the patient’s recognition of a familiar induced discomfort, and referral of said pain (often with motor and autonomic phenomena) to a predictable zone of reference, is referred to as an active MTrP.
In contrast, with a latent MTrP there is no current experience of spontaneous pain, but algesia is inducible with palpation over the tender spot. A latent MTrP may have all the other clinical characteristics of an active MTrP and always has a taut band. Autonomic dysfunction associated with MTrPs includes sweating, persistent lacrimation, coryza, excessive salivation, and pilomotor activities. In addition, there can be proprioceptive disturbances such as imbalance, dizziness, tinnitus, and distorted weight perception of lifted objects.[2]
Several modalities have been studied in an effort to better objectively identify MTrPs. Thermography is a science that records infrared radiation to produce images called thermograms. Based on asymmetric thermal patterns, MTrPs and their referral areas were able to be localized (Figure 3). Others feel that this test is not specific and just finding a “hot” spot on the skin is insufficient to identify a MTrP.[11]
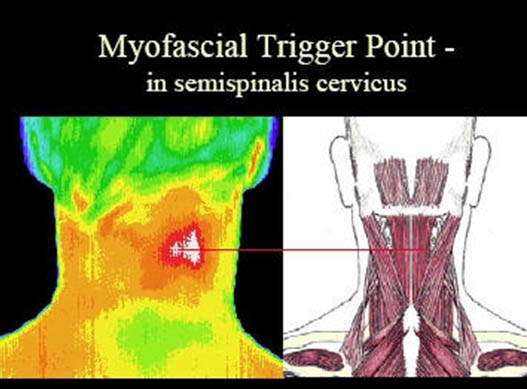
Figure 3. Thermogram of myofascial trigger point. Adapted from dv-2.com.
Needle electromyography (EMG) detects the presence of spontaneous low voltage motor endplate “noise” activity, as well as the high voltage spike activity that is highly characteristic, but not pathognomonic, of MTrPs (Figure 4).[12-13]
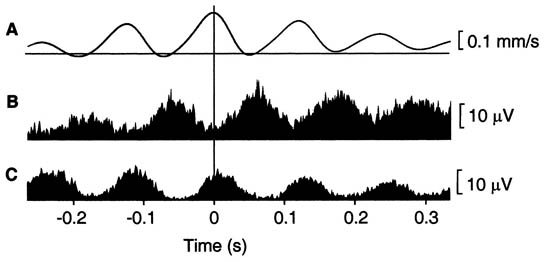
Figure 4. EMG of myofascial trigger Point. Adapted from Proceedings of the National Academy of Sciences.
The use of ultrasound to identify MTrPs holds great promise. In combination with EMG, it has the potential for providing the needed imaging to objectively substantiate the clinical diagnosis of MPS.[14] Measurement of levels of the biochemical substances associated with pain and inflammation in the MTrP region,[15] and magnetic resonance elastography to detect taut band images[16] are potential tools. However, most of these methods require special skills and are relatively expensive at this time.
Pathogenesis
It has been suggested that MPS, as a pain phenomenon, results from the activation of latent MTrPs, as a consequence of certain pathological conditions and predisposing factors such as chronic repetitive minor muscle strain, poor posture, systemic diseases, or neuro-musculoskeletal lesions.[1][17] These triggering pathologic lesions are usually found in regions remote to the latent MTrPs.[18-19]
Exploration of MTrP with needle electromyography (EMG) has shown spontaneous electrical activity (SEA) (Figure 4).[12] Local infusion of phentolamine eliminates this SEA phenomenon and the pain. Based on this finding, it has been suggested that MTrPs have adrenergic dependency.[20-22] Recent studies have clarified the nature of MTrPs.[15][23] In a MTrP region, multiple hyperirritable loci can be found. The sensory components of the MTrP locus are due to sensitized nociceptors that are responsible for pain, referred pain, and local twitch responses. The concentrations of pain and inflammation-related substances such as bradykinin, calcitonin gene-related peptide, substance P, alpha tumor necrosis factor, interleukin 1-beta, serotonin, and norepinephrine are increased in the MTrP region. The motor components are dysfunctional endplates that are responsible for taut band formation as a result of excessive acetylcholine (ACh) leakage. It has been hypothesized that excessive ACh release, sarcomere shortening, and release of the above mentioned substances are three essential features that relate to one another in a positive feedback cycle.
Based on recent human and animal research studies, referred pain and LTR are mediated via a spinal cord mechanism.[1][3-4] They have demonstrated that that the elicited referred pain is secondary to the unmasking of formerly ineffective synaptic connections among neurons corresponding to different receptive fields. A strong noxious stimulus can send the impulse to the corresponding dorsal horn neuron. This induces it to release substance P and calcitonin gene-related peptide, which diffuse to other dorsal horn neurons and increase the efficacy of silent synaptic connections, as a consequence of central sensitization in the spinal cord.
Treatment
It has been strongly suggested that the most important strategy to treat MPS is to identify and treat the underlying etiological lesions appropriately.[17][24-25] MTrPs due to muscle over-activity can be easily inactivated with avoidance of overuse or inappropriate use.
In certain situations, however, treatment (inactivation) of active MTrPs is necessary. This includes cases of intolerable pain, discomfort that interferes with functional activities, persistent pain after elimination of the underlying etiological lesion, and failure in identifying or treating the underlying pathology. Release of muscle tightness due to taut bands may improve the local circulation to facilitate the healing process of the underlying etiological lesion.
Many methods have been used to treat MPS by either treating active MTrPs or their underlying pathology. Conservative treatment should be performed before more aggressive therapy.[17][24] The commonly applied MTrP therapies include intermittent cold and stretch (spray and stretch), topical anesthetic patches,[26-27] deep pressure soft tissue massage,28 trigger point pressure release, post-isometric relaxation, thermotherapy, ultrasound therapy,[29] electrotherapy, and needling (MTrP injection, dry needling, or acupuncture).[2][17][30-31]
With respect to needling, if the needle elicits a LTR, dry needling is as effective as injection of an anesthetic for relief of MTrP symptoms. Conversely, if no LTR occurs, dry needling and injection of anesthetics are equally ineffective.[30] Hence, there is essentially no difference in the effectiveness between dry needling and local anesthetic injection. However, following dry needling, soreness is more likely to occur, lasting longer and more severe.[30] Isotonic saline injection has been found to be equal or even superior to local anesthetic injection.[32-33]
Corticosteroids are used for MTrP injections in support of a theory that chronic mechanical stress to the muscle may produce aspects of an inflammatory reaction. The use of long acting steroids, however, is not recommended for the injection of MTrPs, as they, by themselves, may be destructive to muscle fibers.[34] MTrP injection with BTX-A has been recommended to treat MTrPs.[40-41] However, some recent studies found no significant benefit from BTX-A injection compared with dry needling[42-43] or bupivacaine injection.[44] Considering the cost and risk of BTX-A injections, it is not routinely recommended. The likely mechanism of pain relief provided by needle stimulation is hyperstimulation analgesia.[45] The strong pressure stimulation to the MTrP loci (nociceptors) can provide very strong neural impulses to the dorsal horn cells in the spinal cord, which may then break the vicious cycle of the MTrP circuit.[17][24]
Laser therapy has also been used to treat MTrPs.[35] Snyder-Mackler et al.[36] noted significant pain reduction and an increase in skin resistance after laser therapy. The pain-relieving effect of laser treatment has been proposed by one or a combination of the following mechanisms: circulation enhancement, collagen proliferation, peripheral nerve stimulation, an anti-inflammatory effect, and direct analgesic effect.[37] Shockwave therapy is a newly developed device for treating MTrPs.[38] Hyperbaric oxygen therapy is another novel treatment modality for MPS. Kiralp MZ[39] suggested that tissue hyperoxygenation in trigger points is beneficial. This is predicated upon the theory that trigger points occur in areas of tissue hypoxia resulting from persistent muscle contractions.
Fibromyalgia Syndrome
Introduction
Fibromyalgia syndrome (FMS) is a chronic condition that presents with a constellation of symptoms, including widespread pain, fatigue, dysfunctional sleep and cognitive disruption.[1] The prevalence of this condition, in developed countries, is reported to be 2–12% of the general population. It increases with age, to greater than 7% of those over 70 years of age.[46] Women are 4–7 times more likely to be affected than men. As a consequence, FMS presents as a major financial and social burden to patients, their families, caregivers and to healthcare systems.[47]
Diagnostic Criteria
A multi-center research study has led to the development of research criteria for the classification of FMS by the American College of Rheumatology (Table 2).[48]
| Criterion | Description |
|---|---|
| Adapted from Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology (1990) criteria for the classification of fibromyalgia. Report of multicenter criteria committee. Arthritis Rheum 1990; 33:160-172. | |
| 1. History | Chronic, widespread (four quadrants) soft tissue pain for at least 3 months |
| 2. Examination | Pain (1+ or greater severity) induced by 4 kg of digital palpation pressure at 11 of 18 anatomically defined tender points (TePs) |
| 3. 1 and 2 are true | When 1 and 2 are true, sensitivity and specificity for FM > 80% |
These criteria include a history of widespread pain for at least three months in duration and tenderness elicited by a manual pressure force of 4 kg in at least 11 of 18 anatomically defined tender points (TePs) (Figure 5, Table 3).
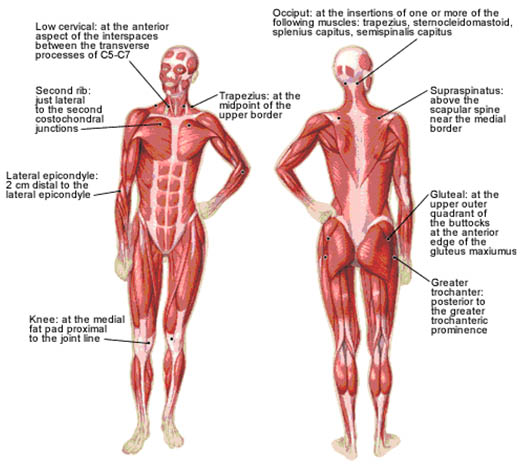
Figure 5. 18 anatomically defined tender points in FMS. Adapted from American Family Physician.
| Number | Official ACR Bilateral Tender Point Sites | |
|---|---|---|
| Adapted from Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology (1990) criteria for the classification of fibromyalgia. Report of multicenter criteria committee. Arthritis Rheum 1990; 33:160-172. | ||
| 1, 2 | Occiput—suboccipital muscle insertions | |
| 3, 4 | Low cervical—anterior aspects of C5-7 intertransverse spaces | |
| 5, 6 | Trapezius—midpoint of upper border | |
| 7, 8 | Supraspinatus—origins above scapular spine, near medial border | |
| 9, 10 | Second rib—upper lateral surface of second costochondral joint | |
| 11, 12 | Lateral epicondyle—2 cm distal to epicondyles | |
| 13, 14 | Gluteal—upper outer buttock, anterior fold of muscle | |
| 15, 16 | Greater trochanter—posterior to trochanteric prominence | |
| 17, 18 | Knees—medial fat pad, just proximal to medial condyle | |
In most patients with FMS, localized or regional pain precedes widespread pain. FMS is often considered to be a rheumatological condition,[49] although there is evidence to support a neurological basis of the pathophysiology. Further, symptom expression in FMS tends to vary on an individual basis, indicative of heterogeneity within the condition and the possibility of subgroups of patients.[50] Patients often present with contributing symptoms of disordered sleep, fatigue, morning stiffness, decreased cognitive function, headache, dizziness, depression, anxiety, chest pains, dysesthesia in the hands, cold intolerance, restless legs, and irritable bowel or bladder syndrome.
The TePs of FMS are physiologically and structurally different from MTrPs of MPS (Table 4).
| Trigger Points of MPS | Tender Points of FMS |
|---|---|
| Adapted from Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology (1990) criteria for the classification of fibromyalgia. Report of multicenter criteria committee. Arthritis Rheum 1990; 33:160-172. | |
|
|
TePs are symmetrically distributed in the body, deep to the skin in various soft tissues, and not necessarily in muscles. Additionally, they show no evidence of histologically abnormal tissues, and TePs do not refer pain to distant locations.
Pathogenesis
The etiology of FMS is still unknown. Over time, multiple theories have been suggested with a transition from a psychiatric process to a muscle disorder, to a genetically predisposed central nervous system dysfunction.[51-53]
Recently, significant progress in the understanding of the pathophysiology (and identification of effective treatments) of FMS has been made.[54] Currently, FMS is described as a condition of heightened generalized sensitization to sensory input manifesting as a constellation of symptoms, including pain. The pathophysiology may include dysfunction of the central nervous system (CNS) pain modulatory systems,[55] dysfunction of the neuroendocrine system and dysautonomia.[56-57] Central sensitization of nociceptive afferent pathways creating a stimulus-independent pain state has been proposed. It is felt that alterations in the level of CNS substance P, the volume of N-methyl-D-aspartate acid receptors, and the CNS mono-aminergic activity levels are responsible for this sensitivity in the FM patient.[58] Many patients with FMS report that other family members have similar chronic painful symptoms, or even a diagnosis of FMS, suggesting a genetic predisposition.[59-60]
Treatment
The management of FMS has been complicated by an overlap of this syndrome with symptoms of other health conditions (e.g., chronic fatigue syndrome, myofascial pain, systemic lupus erythematosus). Although treatment recommendations from the American Pain Society (Table 5) and the European League Against Rheumatism (Table 6) have been published,[61-62] a universally accepted treatment strategy or algorithm is lacking.
| Treatment | Description |
|---|---|
| Adapted from: Burckhardt DC, Goldenberg D, Crofford L, Gerwin R, Jackson K, Kugel P, et al. Guideline for the management of fibromyalgia syndrome pain in adults and children. In APS Clinical Practice Guidelines Series. No 4. Glenview, IL: American Pain Society; 2005. | |
| Pharmacologic Therapies |
Note: Guideline developers considered but did not recommend anticonvulsant medication and hormone therapy including corticosteroids (unless there is concurrent joint, bursa, or tendon inflammation) |
| Nonpharmacologic Therapies |
Note: Guideline developers considered but did not recommend dietary modifications, nutritional supplements, and biologic agents due to lack of sufficient research |
| Multidisciplinary treatment incorporating two or more strategies | Referral to a specialist |
| Recommendation | Description |
|---|---|
| Adapted from: Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008; 67:536-41. | |
| 1. General |
|
| 2. Non-pharmacological management |
|
| 3. Pharmacological management |
|
Current management approaches to improve the overall health status in FMS is based upon a multidisciplinary approach. The objectives are to reduce pain, improve sleep, restore physical function, maintain social interaction, and reestablish emotional balance. To achieve these goals, patients will need rehabilitation, integrating exercise, education (stress management programs, cognitive behavioral therapy (CBT) and pharmacological treatments rather than a classic biomedical model.[54]
Antidepressants, particularly tricyclic antidepressants (TCAs) such as amitriptyline and dothiepin, have been first-line pharmacological therapies for FMS.[63-64] Recently, new selective serotonin reuptake inhibitors, such as paroxetine, and serotonin-norepinephrine reuptake inhibitors, such as duloxetine, milnacipran, venlafaxine have become popular.[65]
Anti-epileptic drugs have also been administered and shown to be effective (or have been used) in the control of symptoms.[66] Both gabapentin and pregabalin block the α2δ subunit of the presynaptic calcium channels leading to a decreased neurotransmitter release and attenuation of abnormal hyperexcitability of the neuronal networks associated with chronic pain.[67] They both improve pain scores and sleep quality. High incidences of adverse effects (e.g., sedation, dizziness) have been observed, though they resolve with ongoing therapy.
Benzodiazepines, which act non-selectively at the BZD1 and BZD2 receptors located on GABAA, have given inconsistent results in clinical trials in patients with FMS. Although benzodiazepines have been shown to be effective in conditions involving neuronal hyperexcitability (e.g., epilepsy and spasticity), and one would assume they would also have the potential of controlling the pain related to the enhanced general sensitization found in FMS, acceptable efficacy has not been observed.[68]
Sodium oxybate, the sodium salt of gamma-hydroxybutyrate, provided significant improvement in the major symptoms of FMS (i.e., pain, tenderness, sleep quality, and fatigue), which has been largely attributed to its capacity to consolidate and improve deep sleep.[69]
Clinical trials of opioid agonists as treatment for FMS have been limited and often ineffective. The effectiveness of opioids in controlling FMS pain may be hindered by the suggestion that these individuals’ central opioid systems are already maximally activated with endogenous endorphins or have a decreased number of central μ-opioid receptors.[70]
Anti-inflammatory medications, such as prednisone and the non-steroidal anti-inflammatory drugs, naproxen and ibuprofen have not borne out to be effective treatments in FMS, suggesting a lack of underlying peripheral structural damage and inflammation.[71]
Good outcomes have been reported with a number of forms of relaxation training including electromyography biofeedback, heart rate variability biofeedback, meditation-based stress reduction, tai-chi, and yoga therapy. In addition, progressive exercise, Jacobson progressive relaxation therapy, and heat applied as a shower or bath can all be self directed treatments. CBT provides improvements in pain-related behavior, coping strategies and overall physical function in patients with FMS.[72]
References
- Hong CZ, Simons DG. Pathophysiologic and electrophysiologic mechanism of myofascial trigger points. Arch Phys Med Rehabil 1998;79:863-872
- Simons DG, Travell JG, Simons LS. Myofascial Pain and Dysfunction: The Trigger Point Manual, vol 1, edn 2. Baltimore: Williams & Wilkins; 1999
- Meese S, Simons DG. Muscle Pain: Understanding Its Nature, Diagnosis, and Treatment. Philadelphia: Lippincott Williams & Wilkins; 2001
- Hong CZ. Algometry in evaluation of trigger points and referred pain. J Musculoskelet Pain 1998;6:47-59
- Sola AE, Rodenberger ML, Gettys BB. Incidence of hypersensitive areas in posterior shoulder muscles. Am J Phys Med 1955;34:585-590
- Kraft GH, Johnson EW, LaBan NM. The fibrositis syndrome. Arch Phys Med Rehabil 1968;49:155-162
- Bonica JJ: Preface. In: Advances in Neurology, Vol. 4. Edited by Bonica JJ. Raven Press, New York, 1974 (p. vii).
- Myburgh C, Larsen All, Hartvigsen J. A systemic, critical review of manual palpation for identifying myofascial trigger points: evidence and clinical significance. Arch Phys Med Rehabil 2008;89:1169-1176
- Kostopoulos D, Nelson AJ, Ingber RS, et al. Reduction of spontaneous electrical activity and pain perception of trigger points in the upper trapezius muscle through trigger point compression and passive stretching. J Musculoskelet Pain 2008;16:266-278
- Gerwin RD, Shannon S, Hong CZ, et al. Identification of myofascial trigger points: interrater agreement and effect of training. Pain 1997;69:65-73
- Kruse RA Jr, Christiansen JA. Thermographic imaging of myofascial trigger points: a follow-up study. Arch Phys Med Rehabil 1992;73:819-823
- Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine 1993;18:1803-1807
- Simons DG, Hong CZ, Simons LS. Spontaneous electrical activity of trigger points. J Musculoske Pain 1995;3(supplement 1):124 (Abstract)
- Gerwin RD, Duranleau D. Ultrasound identification of the myofascial trigger point. Muscle Nerve 1997;20:767-768
- Shah JP, Danoff J V, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil 2008;89:16-23
- Chen Q, Bensamoun S, Basford JR, et al. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil 2007;88:1658-1661
- Hong CZ. Myofascial pain therapy. J Musculoskelet Pain 2004;12:37-43
- Chen SM, Chen J T, Kuan TS, et al. Decrease in pressure pain thresholds of latent myofascial trigger points in the middle finger extensors immediately after continuous piano practice. J Musculoskelet Pain 2000;8:83-92
- Kawakita K, Itoh K, Okada K. Experimental model of trigger points using eccentric exercise. J Musculoskelet Pain 2008;16:29-35
- Simons DG. Myofascial trigger points: The critical experiment. J Musculoskeletal Pain 19997;5:113-118
- Couppe C, Midttun A, Hilden J, et al. Spontaneous needle EMG activity in myofascial trigger points in the infraspinatus muscle: A blinded assessment. J Musculoskeletal Pain 2001;9:7-16
- Rivner MH. The neurophysiology of myofascial pain syndrome. Curr Pain Headache Rep 2001;5:432-440
- Shah JP, Phillips TM, Danoff JV, et al. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Apll Physiol 2005;99:1977-84
- Hong CZ. Treatment of myofascial pain syndrome. Curr Pain Headache Rep 2006;10:345-349
- Gerwin R. Differential diagnosis of trigger points. J Musculoskelet Pain 2004;12:23-28
- Affaitati G., Fabrizio A, Savini A, et al. A randomized, controlled study comparing a lidocaine patch, a placebo patch, and anesthetic injection for treatment of trigger points in patients with myofascial pain syndrome: evaluation of pain and somatic pain thresholds. Clinical Therapeutics 2009;31:705-20
- Hsieh L-F, Hong C-Z, Chern S-H, et al. Efficacy and side effects of diclofenac patch in treatment of patients with myofascial pain syndrome of the upper trapezius. Journal of Pain and Symptom Management, 2009;39:116-125
- Vernon H, Schneider M. Chiropractic management of myofascial trigger points and myofascial pain syndrome: a systematic review of the literature. Council on chiropractic guidelines and practice parameters.
- Srbely JZ, Dickey JP. Randomized controlled study of the antinociceptive effect of ultrasound on trigger point sensitivity: novel applications in myofascial therapy? Clin Rehabil 2007;21:411-417
- Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: the importance of the local twitch response. Am J Phys Med Rehabil 1994;73:256-263
- Yoon S-H, Rah UW, Sheen SS, et al. Comparison of 3 needle sizes for trigger point injection in myofascial pain syndrome of upper and middle trapezius muscle: a randomized controlled trial. Arch Phys Med Rehabil Vol 2009;90:1332-1339
- Sola AE, Kuitert JH. Myofascial trigger point pain in the neck and shoulder girdle. Northwest Med 1955;54:980-984
- Frost FA, Jessen B, Siggaard-Anderson J. A control, double-blind comparison of mepivacaine injection versus saline injection for myofascial pain. Lancet 1980;1:499-501
- Pizzolato P, Mannheimer W. Histopathologic effects of local anesthetic drugs and related substances. Charles C Thomas, Springfield, Ill., 1961 (pp. 40, 41, 60, 71)
- Carrasco T, Guerisoli LD, Guerisoli DM et al. Evaluation of low intensity laser therapy in myofascial pain syndrome. The Journal of Craniomandibular practice 2009;27:243-247
- Snyder-Mackler L, Bork C, Bourbon B, et al. Effect of helium-neon laser on musculoskeletal trigger points. Phys Ther 1986;66:1087-1090
- Chen KH, Hong CZ, Kuo FC, et al. Electrophysiologic effects of a therapeutic laser on myofascial trigger spots of rabbit skeletal muscles. Am J Phys Med Rehabil 2008;87:1006-1014
- Bauermeister W: The diagnosis and treatment of myofascial trigger points using shockwaves. J Musculoske Pain 2004, 12 (Suppl 9):13:365-369
- Kiralp MZ, Uzun G, Dincer U, et al. A novel treatment modality for myofascial pain syndrome: Hyperbaric oxygen therapy. Journal of the National Medical Association 2009;101:77-80
- Cheshire WP, Abashian SW, Mann JD. Botulinum toxin in the treatment of myofascial pain syndrome. Pain 1994;59:65-69
- Wheeler AH, Goolkasian P, Gretz SS. A randomized, double-blind, prospective pilot study of botulinum toxin injection for refractory, unilateral, cervicothoracic, paraspinal, myofascial pain syndrome. Spine 1998;23:1662-1667
- Kamanli A, Kaya A, Ardicoglu 0, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int 2005;25:604-611
- Lew HL, Lee EH, Castaneda A, et al. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Arch Phys Med Rehabil 2008;89:75-80
- Graboski CL, Gray DS, Burnham RS. Botulinum toxin A versus bupivacaine trigger point injections for the treatment of myofascial pain syndrome: a randomized double blind crossover study. Pain 2005;118:170-175
- Melzack R. Myofascial trigger points: relation to acupuncture and mechanism of pain. Arch Phys Med Rehabil 1981;62:114-117
- Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995;38:19-28
- Wolfe F, Anderson J, Harkness D, et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis Rheum 1997;40:1560-1570
- Wolfe F, Smythe HA, Yunus MB, Bennet RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology (1990) criteria for the classification of fibromyalgia. Report of multicenter criteria committee. Arthritis Rheum 1990;33:160-172
- Shir Y, Fitzcharles MA. Should rheumatologists retain ownership of fibromyalgia? [editorial]. J Reumatol 2009;36:667-70
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis: A comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006;54:169-76
- Park DC, Glass JM, Minear M, et al. Cognitive function in fibromyalgia patients. Arthritis Rheum 2001;44:2125-2133
- Ahles TA, Khan SA, Yunus MB, et al. Psychiatric status of patients with primary fibromyalgia, patients with rheumatoid arthritis, and subjects without pain: A blind comparison of DSM-III diagnoses. Am J Psychiat 1991;148:1721-1726
- Pinals RS: Fibromyalgia in the Gilded Age: the case of Alice James. J Clin Rheumatol. 2009;15:427-9
- Goldenberg Dl, Bradley LA, Arnold LM, Gkass J, Clauw D. Understanding fibromyalgia and its related disorders. Prim Care Companion. J Clin Psychiatry 2008;10:133-44
- Russell IJ. Neurochemical pathogenesis of fibromyalgia syndrome. J Musculoskel Pain 1996;1&2:61-92
- Crofford LJ, Pillemer SR, Kalogeras KT, et al. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum 1994;37:1583-1592
- Crofford LJ. Neuroendocrine abnormalities in fibromyalgia and related disorders. Am J Med Sci 1998;315:359-366
- Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003 May;48:1420-9
- Buskila D, Neumann L, Hazanov I, et al. Familial aggregation in the fibromyalgia syndrome. Semin Arthritis Rheum 1996;26:605-611
- Pelligrino MJ, Waylonis GW, Sommer A. Familial occurrence of primary fibromyalgia. Arch Phys Med Rehabil 1989;70:61-63
- Burckhardt DC, Goldenberg D, Crofford L, Gerwin R, Jackson K, Kugel P, et al. Guideline for the management of fibromyalgia syndrome pain in adults and children. In APS Clinical Practice Guidelines Series. No 4. Glenview, IL: American Pain Society 2005
- Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536-41
- Joshi MN, Joshi R, Jain AP. Effect of amitriptyline vs. physiotherapy in management of fibromyalgia syndrome: What predicts a clinical benefit? Postgrad Med 2009;55:185-9
- Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther 2006;8:212
- Patkar AA, Masand PS, Krulewicz S, Mannelli P, Peindl K, Beebe KL, Jiang W. A randomized, controlled, trial of controlled release paroxetine in fibromyalgia. Am J Med. 2007;120:448-54
- Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: Results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;52:1264-1273
- Dooley DJ, Donovan CM, Meder WP, et al. Preferential action of gabapentin and pregabalin at P/Q type voltage sensitive calcium channels: Inhibition of K+-evoked (3H)-norepinephrine release from rat neocortical slices. Synapse 2002;45:171-190
- Hench PK, Cohen R, Mittler MM: Fibromyalgia: Effects of Amitriptilline, Temazepam, and Placebo on Pain and Sleep. Arthritis Rheum 1989;32:S47
- Russel IJ, Perkins AT, Michalek JE: Oxybate for FMS Study Group: Sodium oxybate reliefs pain and improves sleep in fibromyalgia syndrome: A randomized, double-blind, placebo-controlled, multi-center clinical trial. Arthritis Rheum 2009;60:299-309
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27:10000-6
- Yunus MB, Masi AT, Aldag JC. Short term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trial. J Rheumatol 1989;16:527-32
- Burckardt CS: Nonpharmacologic management strategies in fibromyalgia. Rheum Dis Clin North Am 2002;28:291-304
- Ta-Shen Kuan: Current studies on Myofascial Pain Syndrome. Curr Pain & Headache Reports. 2009;13:365-369
- Lawson K. Treatment options and patient perspectives in the management of fibromyalgia: Future trends. Neuropsych Dis Treat 2008; 4:1059-71
- Lawson K. Beneficial treatment of fibromyalgia. J Postgrad Med 2009;55;159-60
Leave a commentOrder by
Newest on top Oldest on top