Intrathecal Dosing and Medication Selection
By Salim Hayek, MD, PhD
Professor, Dept of Anesthesiology
Case Western Reserve University
Chief, Division of Pain Medicine
University Hospitals of Cleveland
Cleveland, OH
Background
Selection of initial intrathecal medication and initial dosing depends largely on the results of the trial. However, it is not unusual for intrathecal medication and dosing to evolve with time after implant. The current intrathecal drug delivery systems (IDDS) are approved by the Food and Drug Administration (FDA) for administration of a single agent and there are only 3 FDA approved agents: baclofen for spasticity and morphine and ziconotide for pain. However, in practice there are other agents used that are considered standard of care and include the opioids hydromorphone and fentanyl, the local anesthetic bupivacaine and the alpha-2 agonist clonidine. And in practice, the vast majority of infusions delivered in IDDS are off-label with also more combination therapy used than monotherapy.1
Best Practice
A number of factors are important in consideration of medication selection. Most importantly, these decisions need to be patient-centric. Factors such as patient’s diagnosis (cancer vs. noncancer related pain), localized vs. diffuse pain, patient’s age and comorbidities and patient’s baseline opioid dosage:
- Patient with cancer or terminal pain may have limited survival.2 Hence, concerns about opioid dose escalation, opioid-induced hyperalgesia and other adverse effects including catheter tip granuloma formation take a secondary seat to pain control
- Patients with localized pain may be candidates for use of the local anesthetic adjuvant bupivacaine which may help also curb opioid dose escalation.3
- Younger patients tend to escalate their opioid dosages faster than older patients.4 This may be of concern in chronic noncancer pain whereby a normal life span may be expected
- Patients on high baseline opioid dosage tend to escalate their opioids at a much faster rate than patients on little or no opioids prior to pump trialing. A number of practitioners now advocate opioid weaning prior to intrathecal trialing.5, 6
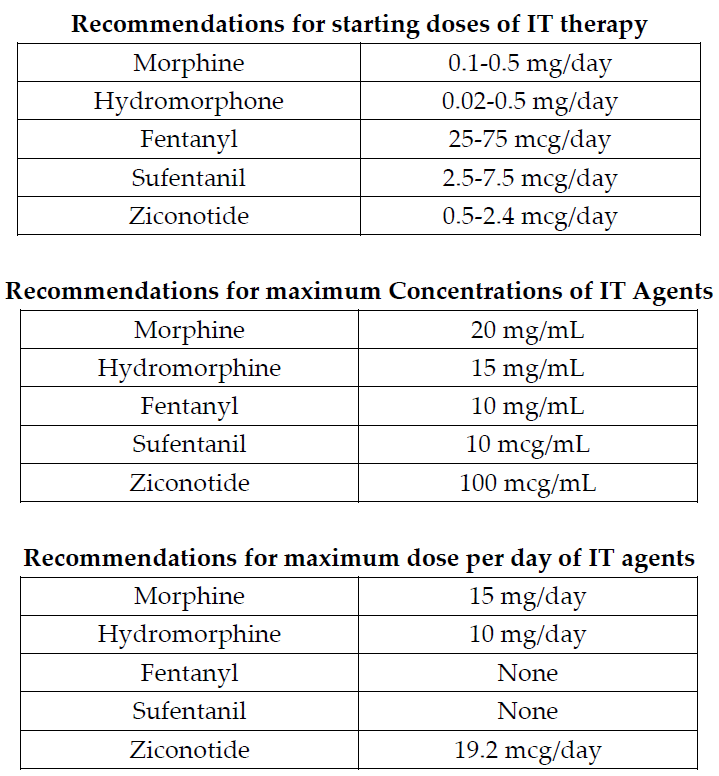
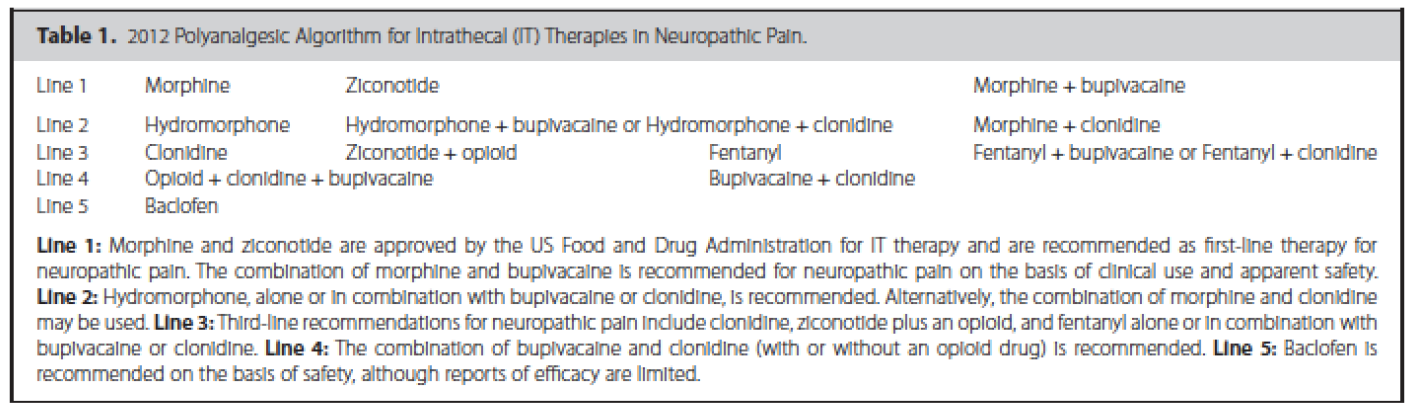
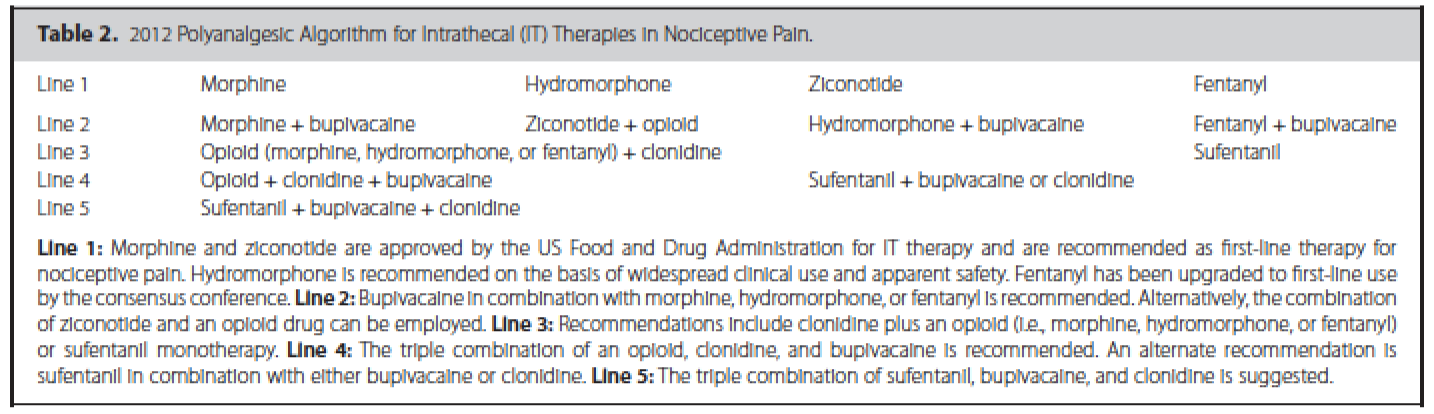
In addition, guidelines have been published that suggest an algorithmic approach to medication choices. The PolyAnalgesic Consensus Conference (PACC) has made recommendations for different lines of therapy for the various medication—including in neuropathic and nociceptive pain.7 In addition, the PACC has published reference ranges for intrathecal medication maximum doses and concentrations. While these recommendations are based mostly on consensus, they act as general guidelines for care of patients considered for intrathecal drug delivery.
References
- Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Current pain and headache reports. 2014;18:388.
- Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-9.
- Veizi IE, Hayek SM, Narouze S, Pope JE, Mekhail N. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-9.
- Hayek SM, Veizi IE, Narouze SN, Mekhail N. Age-dependent intrathecal opioid escalation in chronic noncancer pain patients. Pain Med. 2011;12:1179-89.
- Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain physician. 2011;14:343-51.
- Hamza M, Doleys D, Wells M, Weisbein J, Hoff J, Martin M, et al. Prospective study of 3-year follow-up of low-dose intrathecal opioids in the management of chronic nonmalignant pain. Pain Med. 2012;13:1304-13.
- Deer TR, Prager J, Levy R, Rathmell J, Buchser E, Burton A, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation: Journal of the International Neuromodulation Society. 2012;15:436-64; discussion 64-6.
Leave a commentOrder by
Newest on top Oldest on top