POCUS Spotlight: Lower Extremity DVT Scanning
Cite as: Pulos B, Jones R. POCUS spotlight: lower extremity DVT scanning. ASRA Pain Medicine News 2023;48. https://doi.org/10.52211/asra050123.008.
Introduction
Deep venous thrombosis (DVT) is a common disorder in the hospitalized patient. The failure to diagnose DVTs in a timely fashion can lead to significant morbidity and mortality, especially in critically ill patients. It is considered the leading cause of preventable hospital death in the United States.1 DVTs cannot be ruled out on clinical grounds alone based on the lack of sensitivity of physical examination. Most patients with an identified source of pulmonary embolism will have a proximal lower extremity (LE) DVT. Although most DVTs will begin in calf veins and resolve spontaneously, the problem occurs when a calf DVT extends into the proximal veins. The traditional duplex exam requires accessibility to specialized radiology services and may not be immediately available. Physicians trained in point-of-care ultrasound (POCUS) LE DVT studies can perform these studies efficiently and with a high degree of sensitivity and specificity. A 2013 meta-analysis of emergency medicine physicians performing POCUS studies for DVT detection found a pooled sensitivity of 96% and specificity of 97%.2 The venous compression ultrasound examination has been shown to have similar accuracy and therefore is an important skill for anesthesiologists to learn.
Indications
- Clinical suspicion of LE DVT
- Patients may present with unilateral leg pain and swelling.
- Patients may have a history of recent surgery, trauma, or immobilization.
- Clinical suspicion of pulmonary embolism
- Component of POCUS shock protocol
Lower extremity DVTs cannot be ruled out on clinical grounds and can be associated with significant morbidity and mortality.
Acquisition
Protocol
There are multiple scanning protocols, each of which has its own pros and cons. The whole-leg protocol with color and spectral Doppler is time consuming and technically challenging since the calf veins are included. Distal LE DVTs have a lower risk of embolization, and there is a lower sensitivity of compression ultrasound to detect isolated calf vein DVTs. Therefore, this protocol is not utilized as a POCUS scanning protocol and will not be discussed in this article.
POCUS exams to detect DVT aim to find a lack of compressibility in a deep vein. POCUS LE DVT protocols focus on the proximal LE veins and include two-point, three-point, and continuous proximal LE protocols. While the two-point and three-point protocols have good sensitivity for the detection of proximal LE DVTs, there is a small risk of missing focal proximal LE DVTs.3,4 Therefore, it is the authors’ preference to utilize a proximal scanning protocol that includes continuous scanning with intermittent compression at 1- 2 cm intervals from the common femoral vein (CFV) through the trifurcation of the popliteal vein (PV) and that protocol will be discussed in this article.
Patient Positioning/Preparation
- Reverse Trendelenburg position to promote venous distention (if not contraindicated).
- Leg should be placed in external rotation for scanning the CFV and femoral vein (FV).
- Leg should be placed with knee flexed for scanning the PV.
- Ultrasound gel should be placed from the groin to the adductor canal (Figure 1).
Figure 1. Ultrasound gel placement on lower extremity for the common femoral vein (CFV) through femoral vein (FV) portion of the proximal lower extremity deep vein thrombosis (LE DVT) scan.
Transducer Selection
High-frequency linear transduucer (12-5 MHz) is preferred, but lower-frequency curvilinear (5-2 MHz) may be needed in larger patients where required scanning depth is > 6 cm.
LE Venous Anatomy
The proximal LE venous system consists of the external iliac vein (EIV), CFV, FV, profunda femoral vein (PFV) and PV (Figure 2).
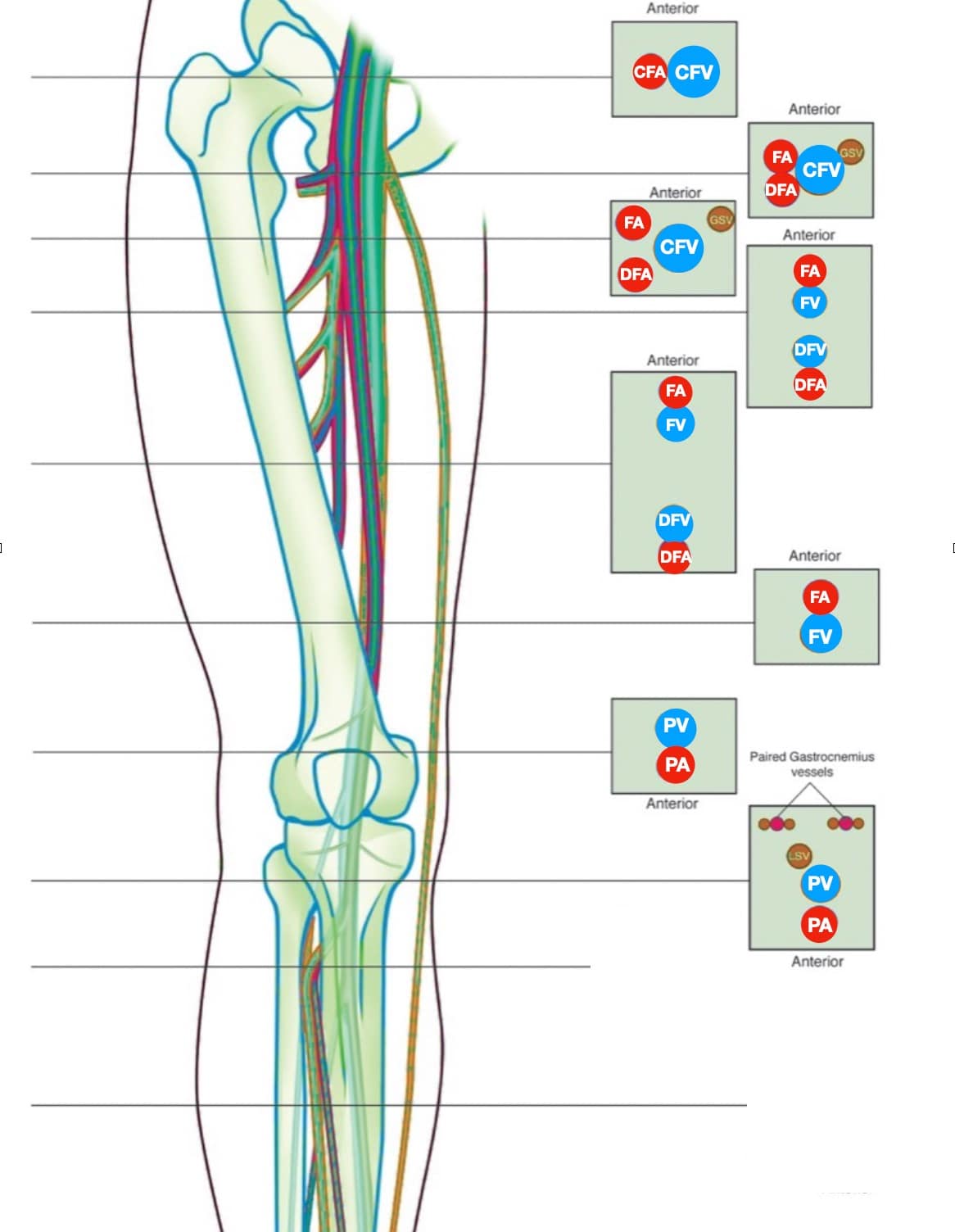
Figure 2. The lower extremity (LE) venous system. Note that the direction of the proximal examination goes in a caudal and medial direction following the veins.
CFA = common femoral artery, CFV = common femoral vein, DFA = deep femoral artery, DFV = deep femoral vein, FA = femoral artery, FV = femoral vein, GSV = greater saphenous vein, LSV = lesser saphenous vein, PA = popliteal artery, PV = popliteal vein
Proximal Exam
Due to its location in the pelvis, the EIV can be difficult to visualize, so the proximal protocol begins with the transducer in the transverse position high in the groin, just distal to the inguinal ligament with the CFV at the saphenous vein (SV) branch point visualized (Figure 3). A normal vein will be anechoic and easily compressible (Figure 4). Compression should be performed with the transducer transverse to the vein with compression being directed in an anterior-posterior direction. Compression should not be performed in a longitudinal plane to the vein since false-positive results can occur from “side-walling” and sliding off the vein, which can appear sonographically as a true compression. Tenting of the artery means that more than enough pressure has been applied.
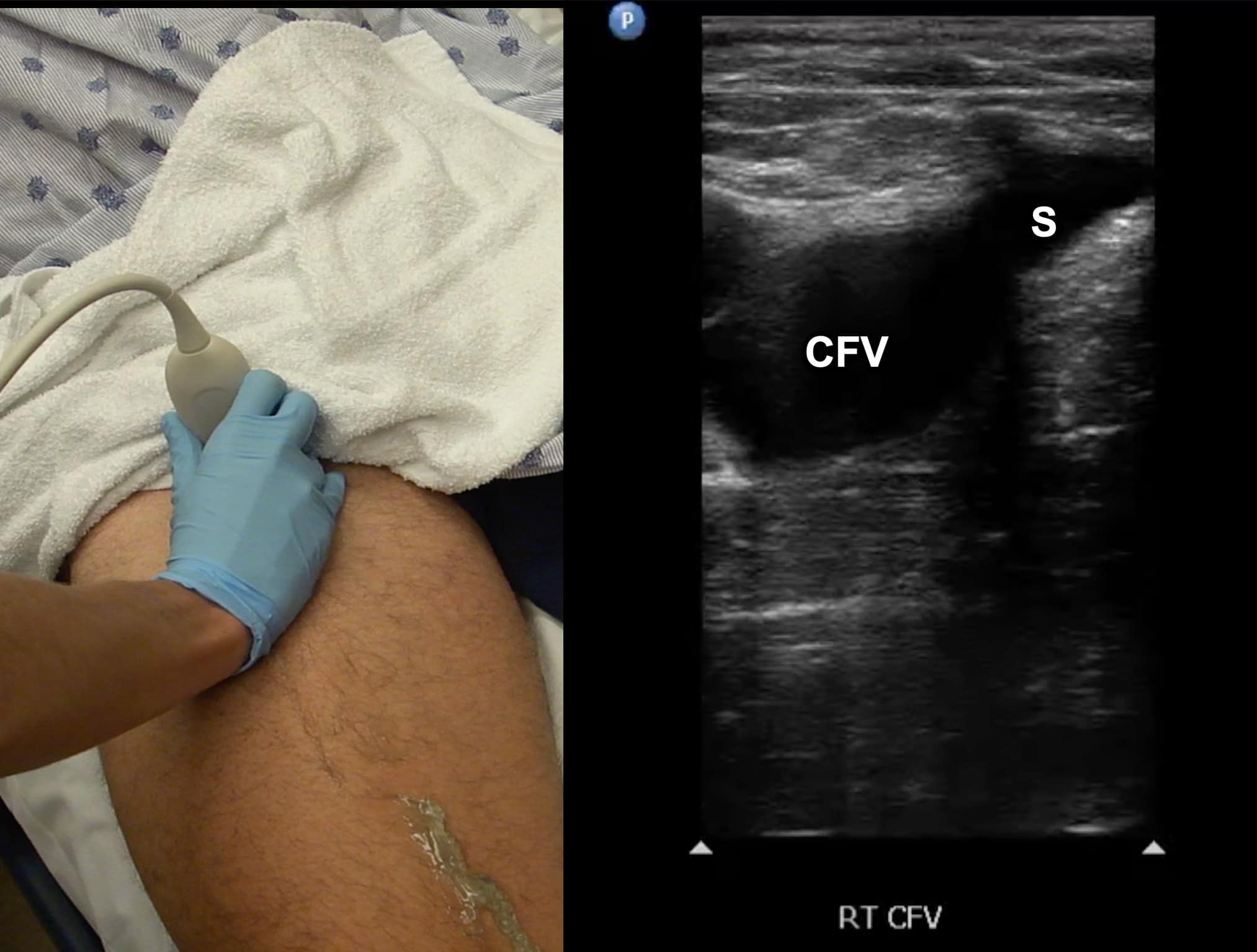
Figure 3. Split screen demonstrating transverse window of common femoral vein (CFV)/greater saphenous vein (S) junction with transducer position (indicator toward patient’s right).
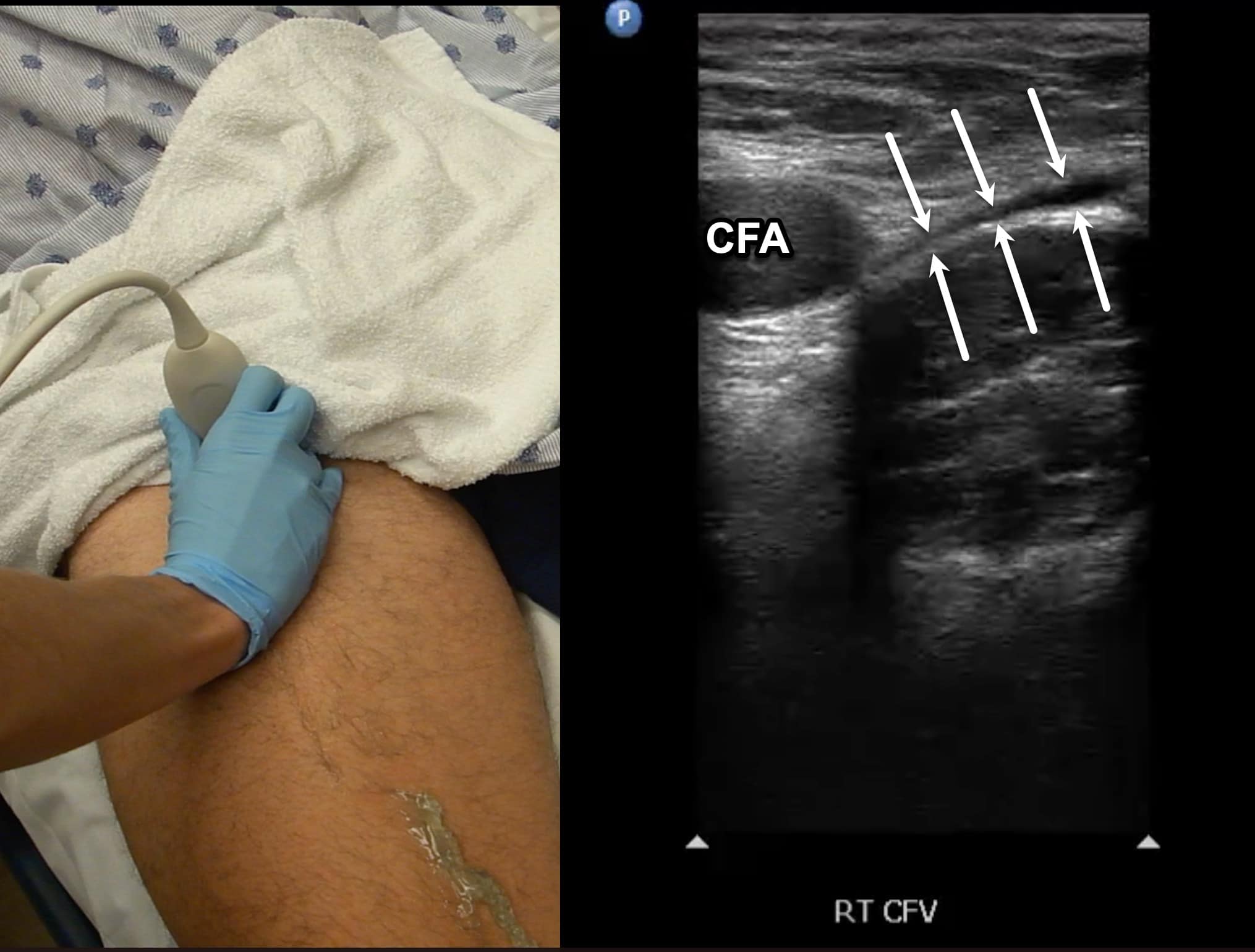
Figure 4. Split screen demonstrating compression of the common femoral vein (CFV) (between arrows) with transducer position shown. Note the common femoral artery (CFA) lateral to the CFV.
The transducer is directed in a caudal direction following the CFV with intermittent compressions performed at 1-2 cm intervals looking for lack of compressibility or evidence of intraluminal echogenicity. The common femoral artery (CFA) will be noted first to divide into the femoral artery (FA) and profunda femoral artery (PFA) (Figure 5). The CFV is followed until it divides into the FV and the PFV with intermittent compressions continued (Figure 6). The PFV is an unlikely location of isolated LE DVT and does not need to be followed.
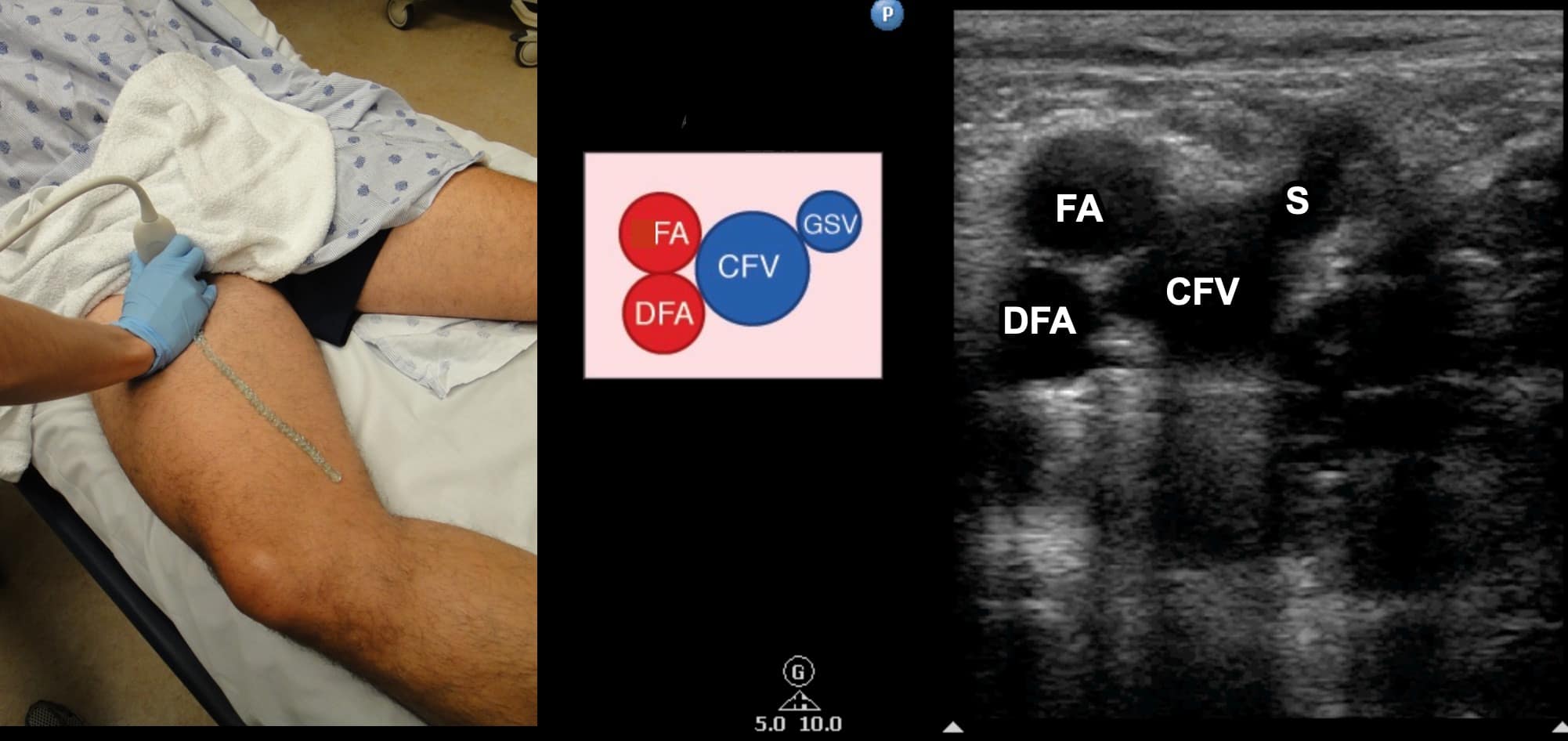
Figure 5. Split screen demonstrating transverse window of femoral artery (FA) and deep femoral artery (DFA) along with common femoral artery (CFV) and saphenous (S) vein with transducer position shown.
CFA = common femoral artery, DFV = deep femoral vein, FV = femoral vein, GSV = greater saphenous vein, LSV = lesser saphenous vein, PA = popliteal artery, PV = popliteal vein
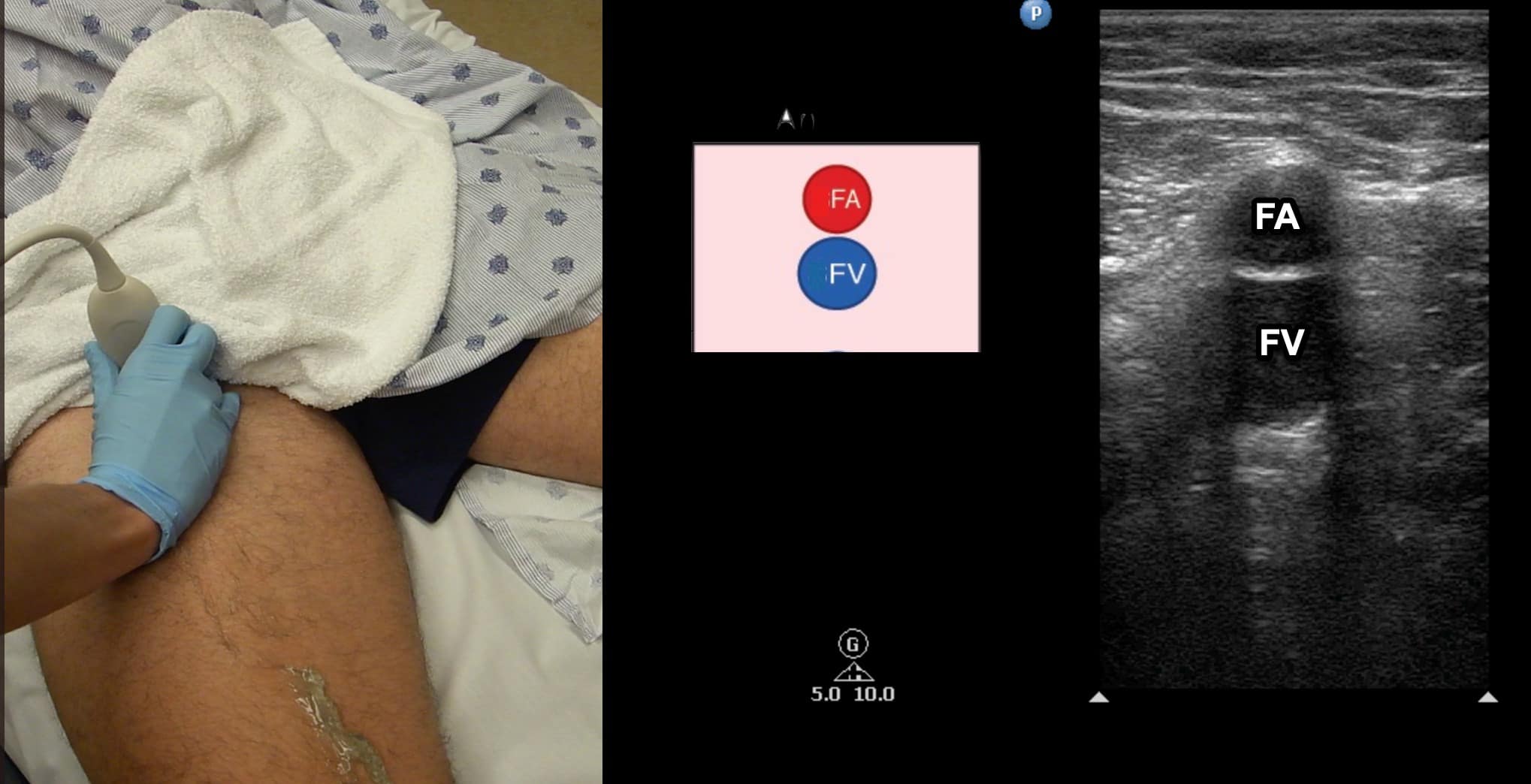
Figure 6. Split screen demonstrating transverse window of femoral vein (FV) with femoral artery (FA) located anterior. Transducer position shown.
Once at the CFV/FV junction, it is important to look for evidence of venous duplication. Venous duplication occurs in a significant number of patients and is seen most commonly in the region of the FV and PV (Figure 7). Failure to identify venous duplication could lead to missing a DVT that occurs in only one of the systems. The FA is seen anterior to the FV in this region (Figure 8). As the FV approaches the adductor canal, an anterior approach utilizing an increased field of depth can also be used to better visualize the vein. Compression at this level is done by placing the freehand posteriorly and squeezing since transducer pressure will not result in venous compression with this approach (Figure 9).
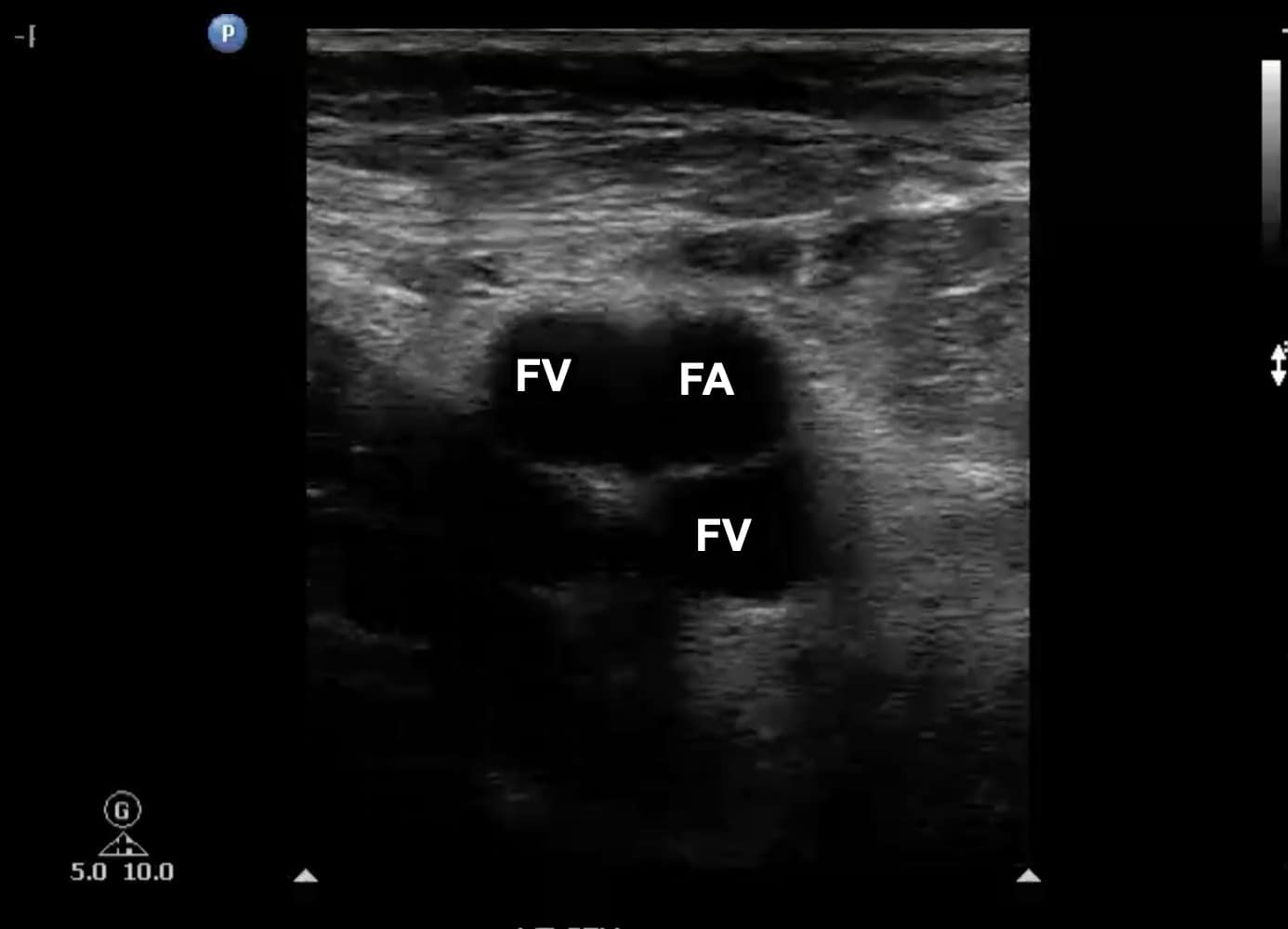
Figure 7. Transverse window at level of left femoral vein (FV) with duplication noted. Note that one branch is medial to the femoral artery (FA) and the other is posterior.

Figure 8. Split screen demonstrating transverse window of the mid-femoral vein (FV). Note that the FV is located deep to the femoral artery (FA). The deep femoral artery (DFA) and deep femoral vein (DFV) can no longer be visualized.
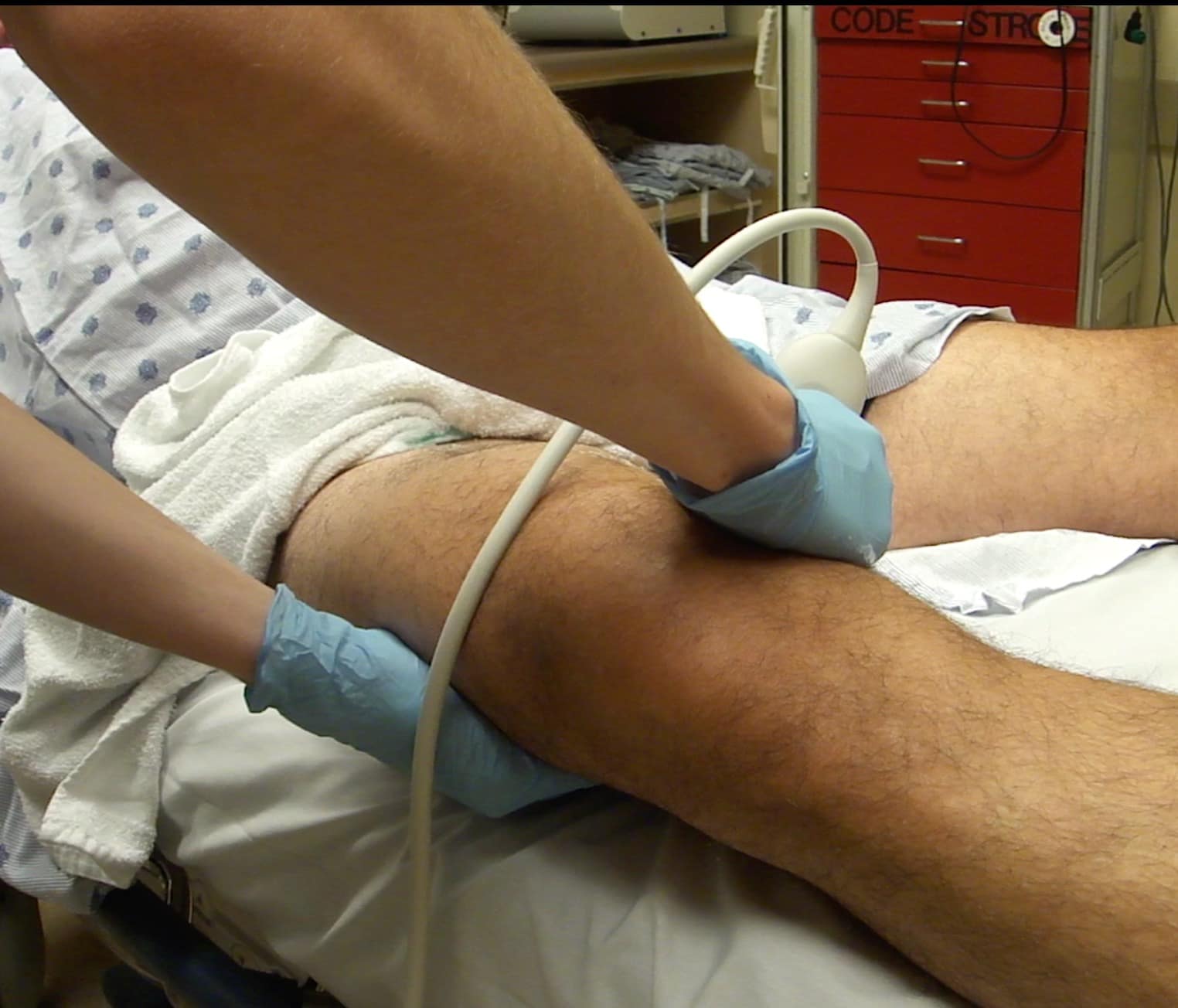
Figure 9. Two-hand technique for compressing the distal femoral vein (FV) in the adductor canal. An anterior or medial scanning approach can be utilized. Note the freehand that will be compressing is placed on the posterior aspect of the leg.
The PV is visualized with the knee flexed to about 30 degrees so the transducer can be placed in the posterior fossa (Figure 10). The popliteal vein is located anatomically posterior to the popliteal artery. Note that since scanning is from the back of the leg, the PV will be noted in the near field (Figure 11). The PV should be followed and compressed at intervals until the popliteal vein trifurcates. The examination is completed at this point. (See video for scanning protocol.)
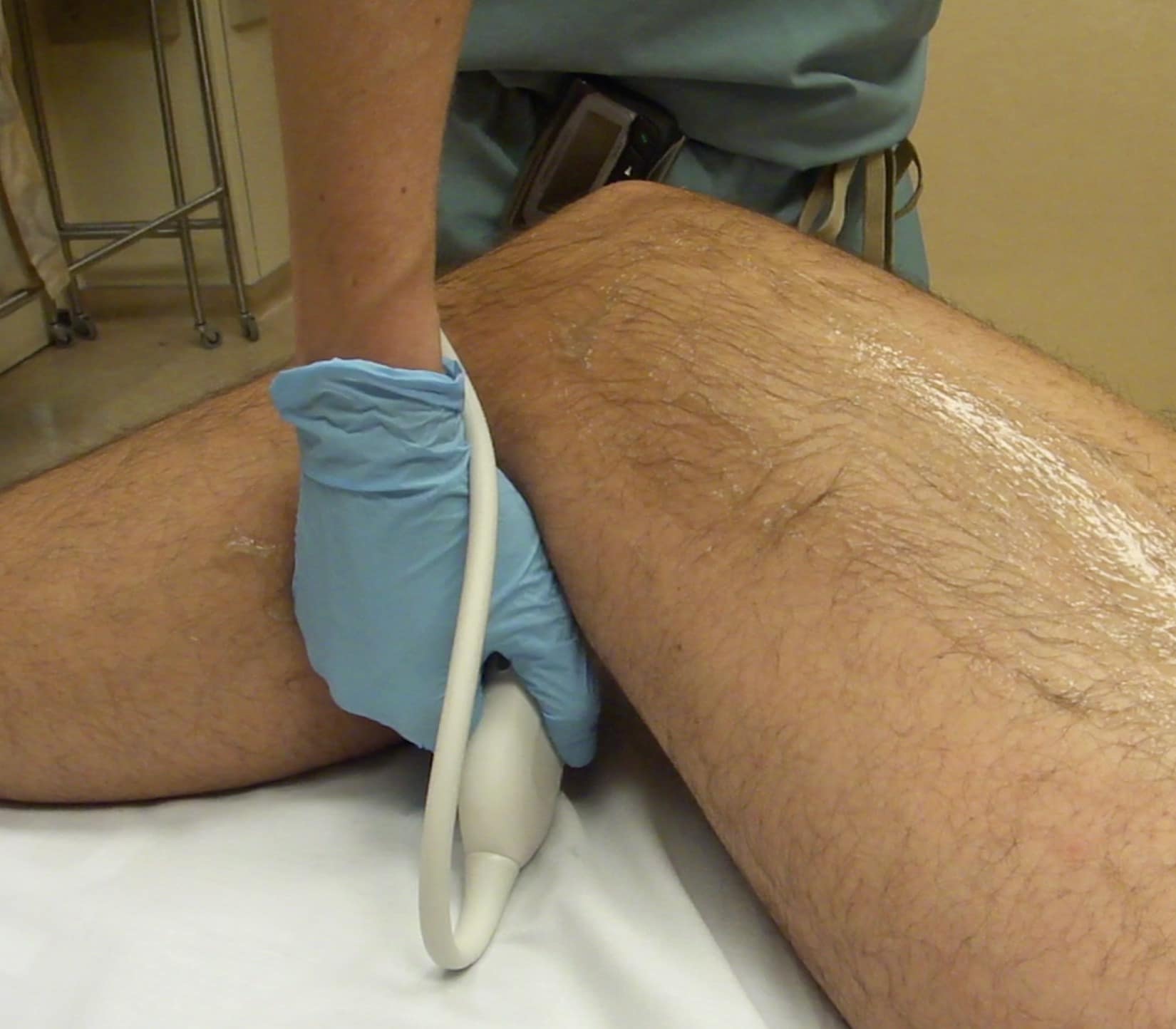
Figure 10. Transducer placement for evaluation of the popliteal vein (PV). The leg is flexed to approximately 30 degrees. The transducer indicator is directed toward the patient’s right.
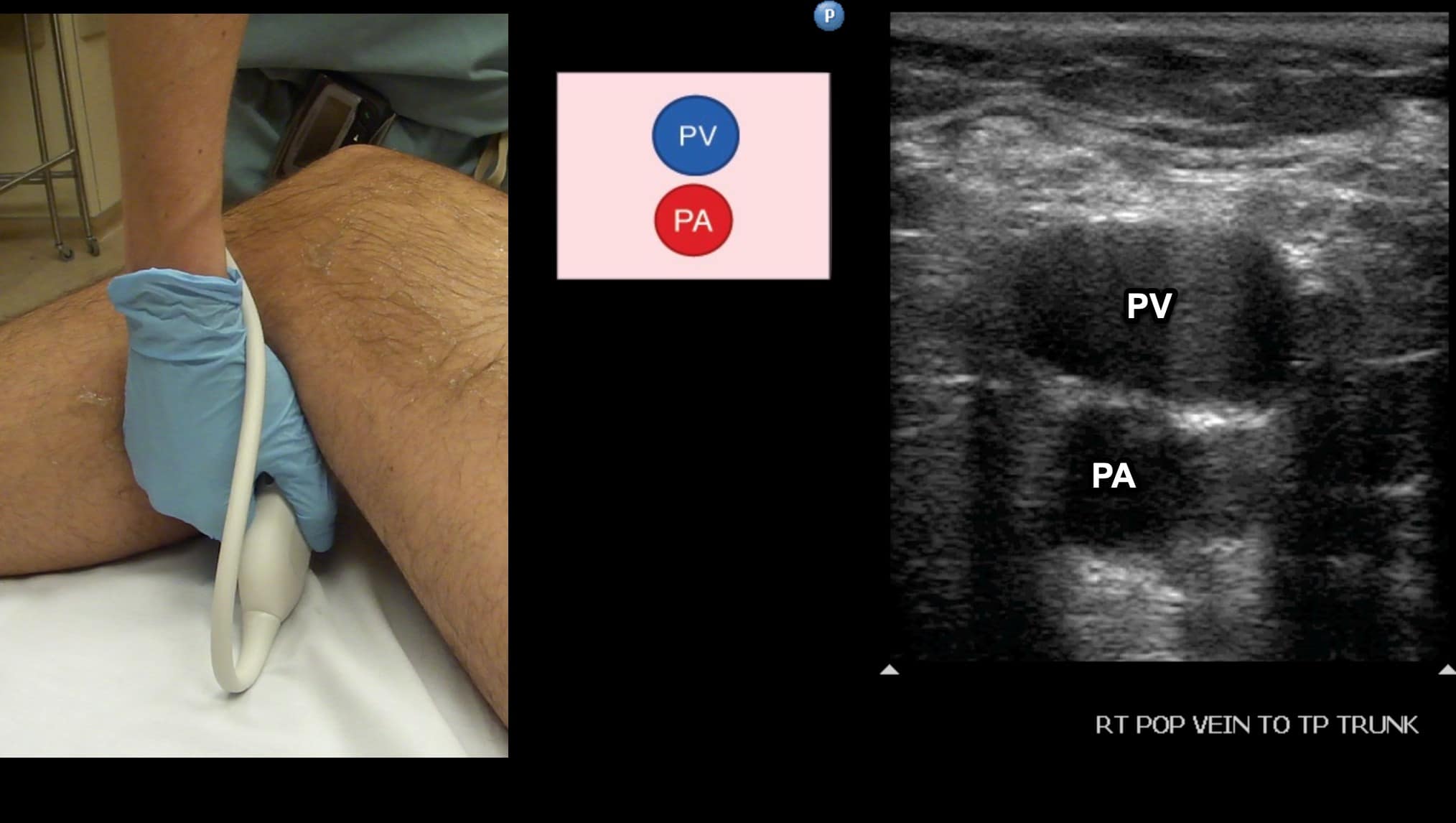
Figure 11. Split screen demonstrating transverse window of the popliteal vein (PV). Note that since we are scanning in a posterior to anterior direction that the PV is seen in the near field even though it is anatomically posterior to the popliteal artery (PA).
The use of Doppler imaging can be used on a selected basis but is not a required component of the proximal LE compression ultrasound. The routine use of augmentation has not been shown to improve sensitivity or specificity for the detection of proximal LE DVTs.5,6 When a proximal iliofemoral DVT is suspected, spectral Doppler of the CFV can be performed to assess for respiratory variation (Figure 12). Respiratory variation will be absent in patients with complete iliofemoral occlusion.
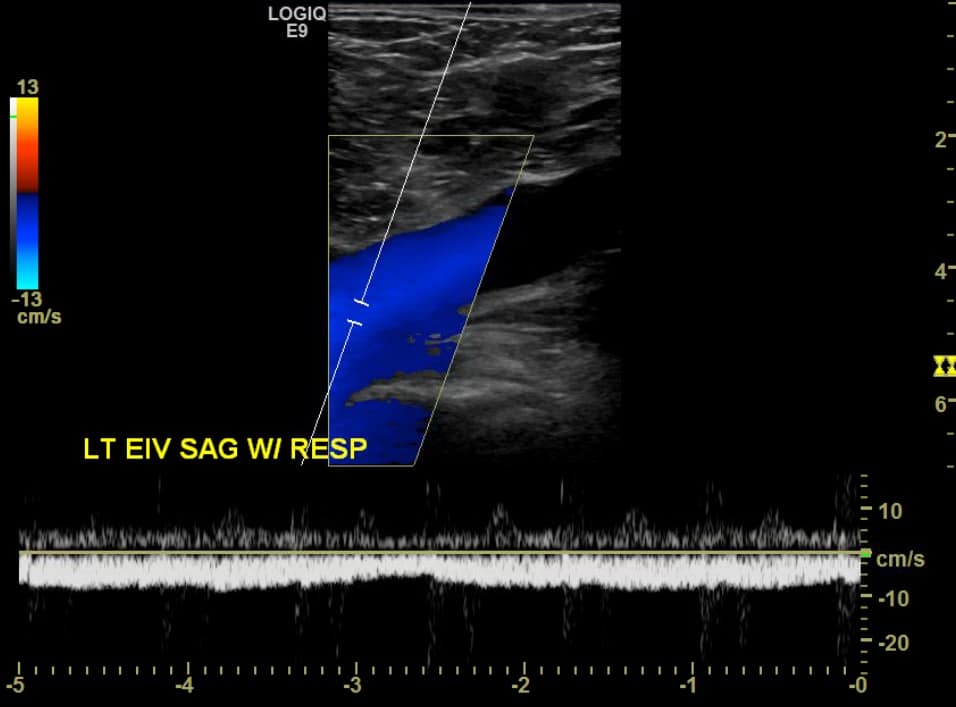
Figure 12. Sagittal window of common femoral vein (CFV) with spectral Doppler demonstrating normal respiratory variation.
Interpretation
The goals of the compression ultrasound study are to detect the presence or absence of thrombus through grayscale findings and the lack of compression. The sonographic characteristics of acute thrombus include homogeneous echogenic appearance, lightly speckled soft/dark echoes, partial or no compressibility, incomplete adherence to wall noted on long-axis, and venous distention (Figures 13 and 14). The ability to differentiate acute from chronic thrombus can be challenging even for highly skilled sonologists, and a complete discussion is beyond the scope of this article.
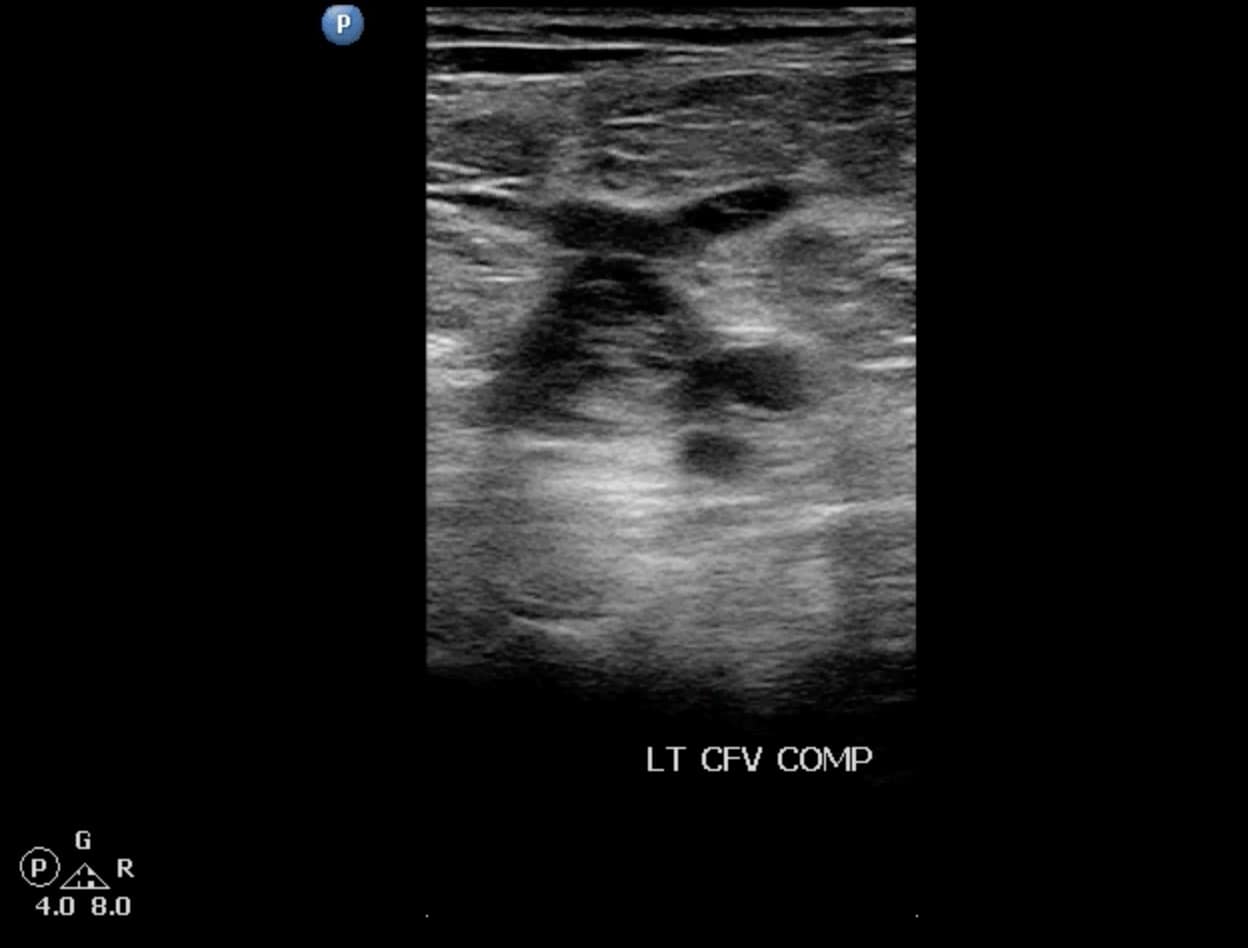
Figure 13. Acute deep vein thrombosis (DVT) of the left common femoral vein (CFV) seen on transverse window. Note the venous distention with intraluminal echoes.
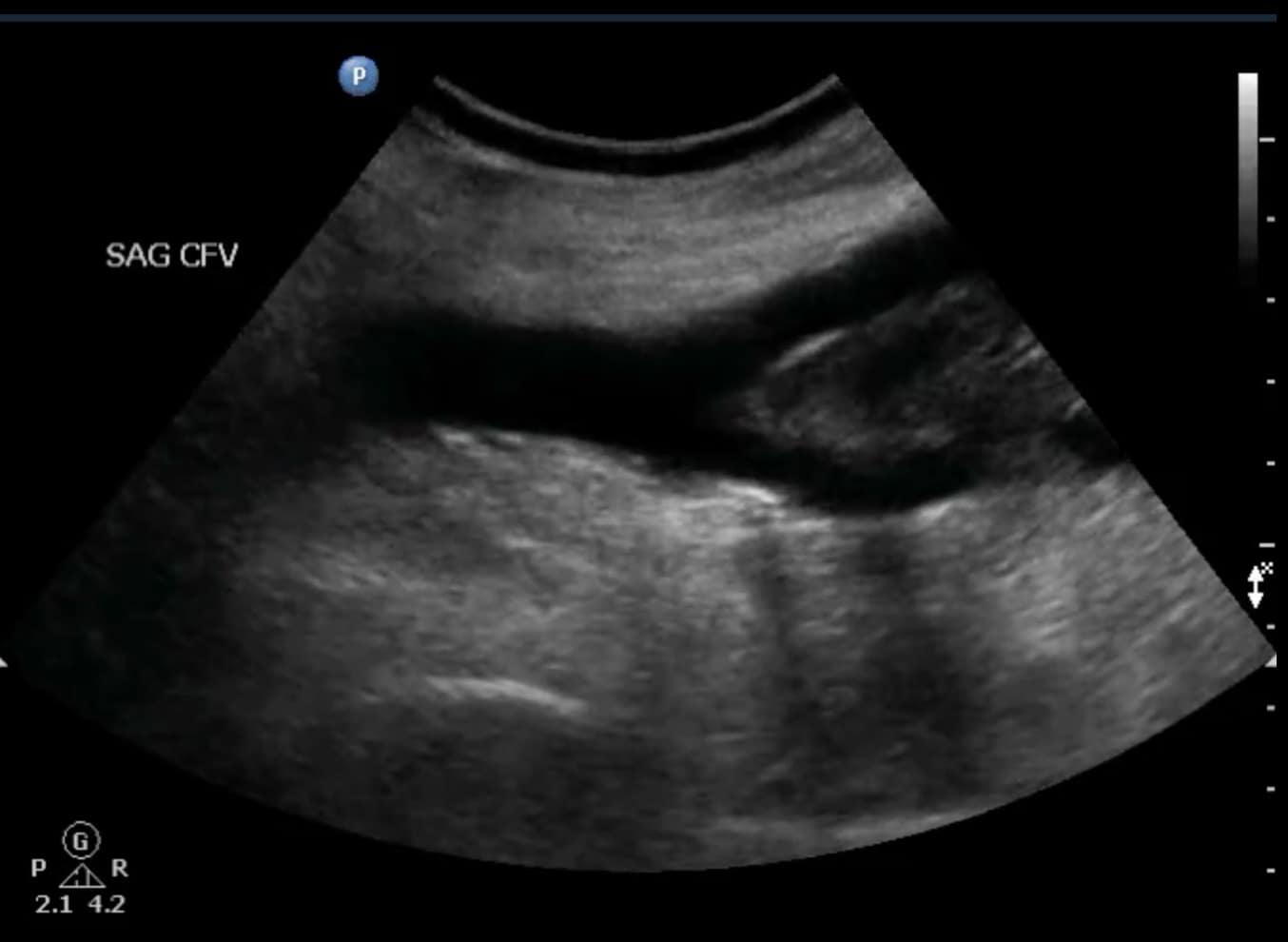
Figure 14. Sagittal window of common femoral vein (CFV) showing proximal edge of an acute deep vein thrombosis (DVT). Note that the proximal portion is free and not adhered to the wall. This patient was scanned using a curvilinear transducer.
Structures, such as lymph nodes or nerves, can be confused with a DVT. However, lymph nodes will be a fixed length and not associated with an adjacent artery (Figure 15). Nerves will often appear as cylindrical, hyperechoic structures with the characteristic “honeycomb” appearance. Baker’s cysts can also be seen during performance of this examination while evaluating the PV. These cysts are located between the medial head of the gastrocnemius and semimembranosus tendons and may be anechoic or echogenic with a characteristic beak-like appearance on sagittal imaging, but notably will not exhibit blood flow within (Figure 16).
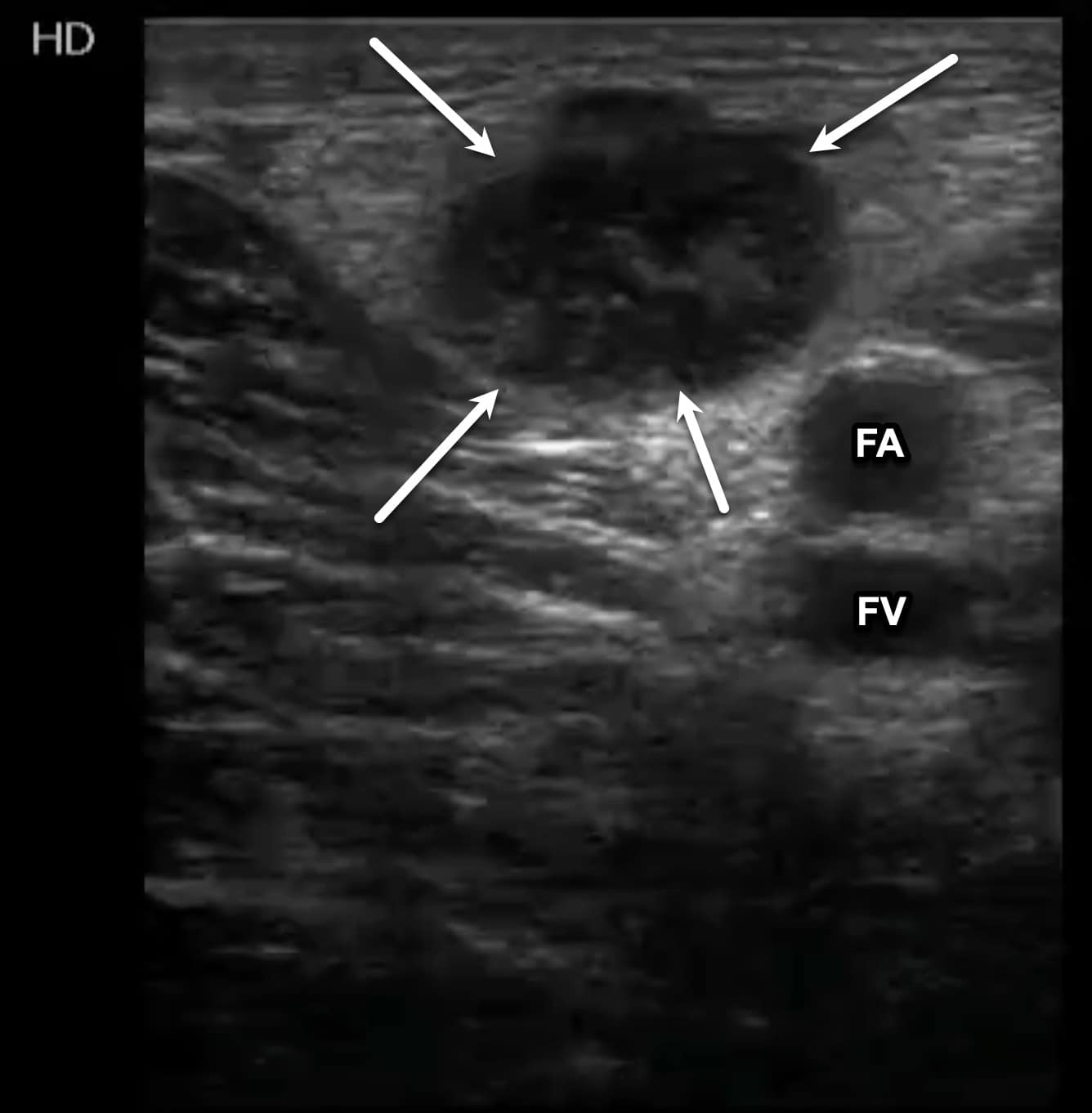
Figure 15. Transverse window of an enlarged inguinal lymph node (arrows). The lymph node has an echogenic, circular appearance like that of a deep vein thrombosis (DVT). The femoral artery (FA) and femoral vein (FV) can be seen deep to the lymph node.
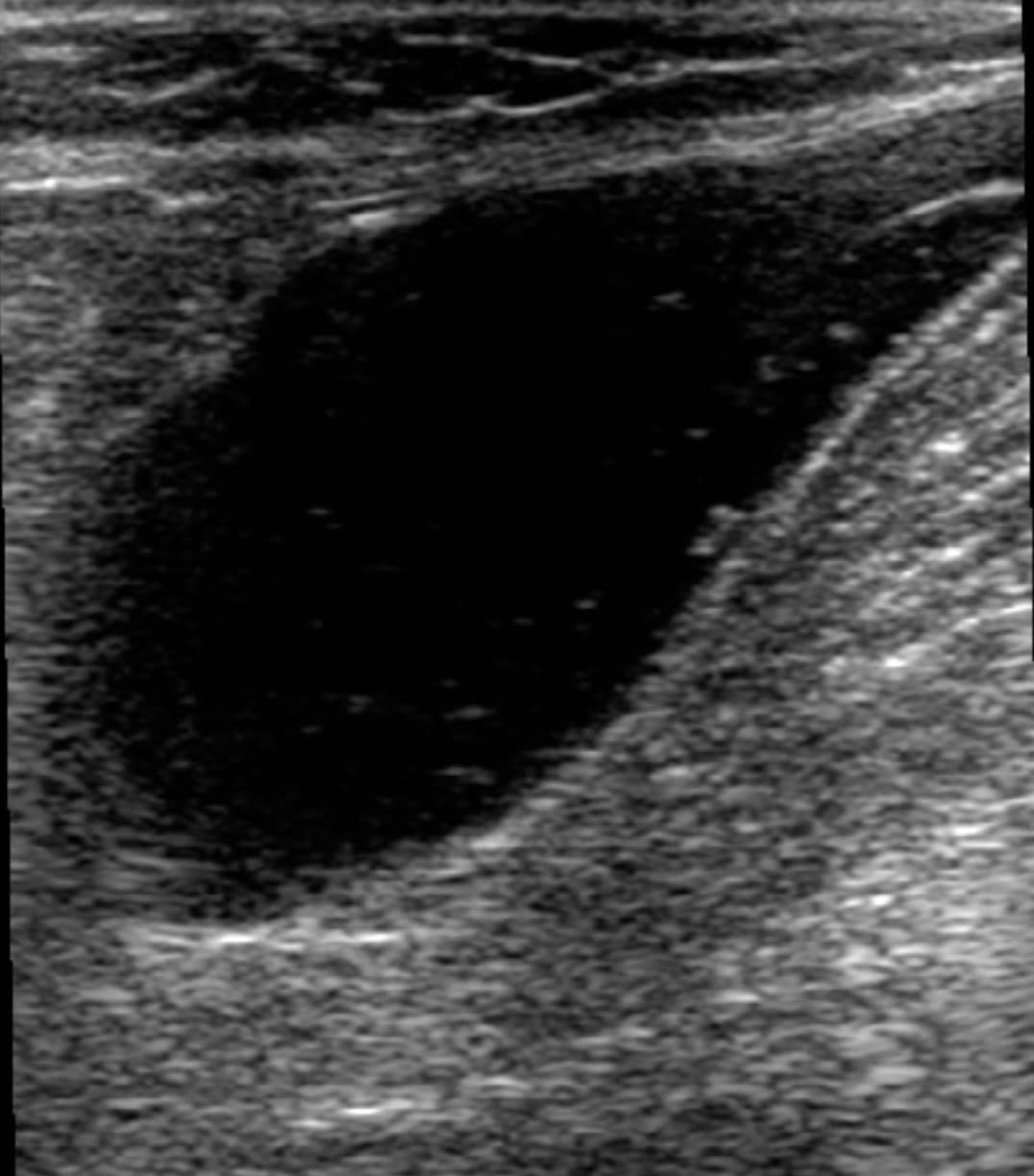
Figure 16. Sagittal window of a Baker’s cyst. Note the beaklike appearance of the neck.
Medical Decision Making
The POCUS proximal DVT exam can accurately rule out the presence of a proximal LE DVT. Isolated calf DVTs are not ruled out by this protocol. Importantly, the absence of a proximal LE DVT does not rule out the presence of a pulmonary embolism.
The POCUS DVT exam is particularly useful in the high-risk patient when a delay in diagnosis while waiting for a radiology-performed exam would affect outcomes. It is also helpful in patients who are at increased risk from anti-coagulation (such as post-operative patients), and the DVT exam can help to inform the risks and benefits discussion of starting treatment.
Follow-up studies of POCUS proximal DVT exams are often recommended, both to confirm the presence or absence of DVT and to monitor progression.
Summary
Lower extremity DVTs cannot be ruled out on clinical grounds and can be associated with significant morbidity and mortality. Studies have shown that lower extremity venous compression examinations can be accurately performed by POCUS users after limited training. The proximal LE DVT venous compression protocol is a high-yield examination that can accurately diagnose proximal DVTs without the need for Doppler interrogation. The ability to perform this examination rapidly at the bedside in the perioperative or critical care setting can have a dramatic impact on patient care.
Bridget Pulos, MD, FASA, is the program director for anesthesiology residency and an assistant professor of anesthesiology and perioperative medicine at the Mayo Clinic in Rochester, MN.
Robert Jones, DO, FACEP, FAIUM, is the system-wide clinic ultrasound chair for the MetroHealth System and a professor of emergency medicine at Case Western Reserve University, both in Cleveland, OH.
References
- Maynard GA, Stein JM. Preventing Hospital-Associated Venous Thromboembolism: A Guide for Effective Quality Improvement. Rockville, MD: Agency for Healthcare Research and Quality, United States Department of Health and Human Services; 2008.
- Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency physician-performed ultrasonography in the diagnosis of deep-vein thrombosis: a systematic review and meta-analysis. Thromb Haemos 2013;109(1):137-45. https://doi.org/10.1160/TH12-07-0473
- Tabbut M, Ebersole N, Icken L, et al. Two-point compression ultrasound technique risks missing isolated femoral vein DVTs. West J Emerg Med 2022;23(4):597-600. https://doi.org/10.5811/westjem.2022.2.53830
- Adhikai S, Zeger W, Thom C, et al. Isolated deep vein thrombosis: implications for 2-point compression ultrasonography of the lower extremity. Ann Emerg Med 2015;66(3):262-6. https://doi.org/10.1016/j.annemergmed.2014.10.032
- Lockhart M, Sheldon H, Robbin M. Augmentation in lower extremity sonography for the detection of deep venous thrombosis. AJR Am J Roentgenol 2005;184(2):419-22. https://doi.org/2214/ajr.184.2.01840419
- McQueen AS, Elliott ST, Keir MJ. Ultrasonography for suspected deep vein thrombosis: how useful is single-point augmentation? Clin Radiol 2009;64(2):148-55. https://doi.org/1016/j.crad.2008.07.014

