A Primer for Pain Physicians on Gadolinium-Based Contrast Agents: Caution Is Advised
Cite as: Pellis Z, DeRiggi L, Shaftner K, Provenzano DA. A primer for pain physicians on gadolinium-based contrast agents: caution is advised. ASRA News 2020;45. https://doi.org/10.52211/asra110120.074
Gadolinium-based contrast agents (GBCAs) are approved by the Food and Drug Administration (FDA) for intravenous use during MRI examinations.[1] In the field of interventional pain medicine, GBCAs have been suggested as an off label alternative to iodine-based contrast agents in patients with a documented iodine allergy. [2,3] Recorded interventional pain procedures for which GBCAs have been used as a visualization agent include discograms, epidural steroid injections, nerve blocks, facet injections, minimally invasive lumbar decompression, and intrathecal pump studies. [2,3]
Intrathecal gadolinium poses a serious risk of neurotoxicity, even at small doses.
When used for FDA-approved indications, GBCAs are considered relatively safe, with known potential adverse events including acute hypersensitivity, physiologic reactions (eg, pain at the injection site, nausea, headache), nephrogenic systemic fibrosis in patients with impaired renal function, encephalopathy, gadolinium storage condition, and gadolinium deposition disease. [4] However, less attention has been given to the risks associated with using GBCAs off-label in interventional pain procedures. Both intentional and unintentional delivery of a GBCA into the intrathecal space pose a risk of severe acute neurotoxicity. Acute toxicity with intrathecal administration may include neurologic and cardiovascular sequelae of seizures, confusion, nausea, tachycardia, fever, respiratory decline, and ultimately death (Table 1). [5]
Table 1. Previous cases of intrathecal gadolinium neurotoxicityΦ
Author | Procedure | GBCA* dose used | Signs and Symptoms |
Arlt et al. [24] | CT myelogram | Gadopentetate dimeglumine 20 mL 7 µmol/g brain**, Ψ | Confusion, nausea, vomiting, dysarthria, somnolence, blurred vision, delirium, limb ataxia, gaze-evoked nystagmus, aggressive behavior, visual and auditory hallucination, incomplete anterograde amnesia |
Li et al. [25] | MRI myelography | Gadopentetate dimeglumine 15 mL 5.35 µmol/g brain**, Ψ | Headache, nausea, vomiting, coma, systemic seizures |
Kapoor et al. [26] | Epidural steroid injection | Gadodiamide 4 mL 1.43 µmol/g brain** | Mental status changes, grand-mal seizure, respiratory distress, agitation, hyperglycemia, sinus tachycardia, respiratory acidosis, metabolic alkalosis, amnesia |
Park et al. [27] | CT myelogram | Gadopentetate dimeglumine 6 mL 2.14 µmol/g brain**, Ψ | Confusion, global aphasia, vomiting, stupor, severe rigidity, intermittent seizures, fever, high blood pressure |
Nayak et al. [28] | Administered through side port of an intraventricular catheter | Gadopentetate dimeglumine 10 mL 3.57 µmol/g brain** | Agitation, labile blood pressure, aphasia, dysarthria, depressed mentation, right facial droop, increased urine output |
Reeves et al. [29] | Intrathecal catheter contrast study | Gadobutrol 2 mL 1.43 µmol/g brain | Severe spastic pain, spasms in lower extremities |
Popescu et al.[22] | L4-L5 interlaminar epidural steroid injection | Gadobutrol 1.5 mL 1.07 µmol/g brain** | Vomiting, seizure activity, impaired consciousness and respiratory compromise requiring intubation |
Provenzano et al.[5] | Minimally invasive lumbar decompression | Gadoteridol 5 mL 2.3 µmol/g brain** | Seizure, mental status changes, severe headache, apnea, agitation, fever, increased muscle tone, eye and tongue twitching, wide-complex pulseless tachycardia, multisystem organ failure, death |
*GBCA – gadolinium-based contrast agent. In this column, volume of GBCA administered is provided alongside the estimated concentration of gadolinium per gram of brain. Calculated using 1400 g as the average weight of the human brain in order
to be consistent with calculations from Arlt et al.[24]
**Intrathecal administration was unintentional.
Ψ Unintentional intrathecal administration was due to drug error.
Φ Adapted from Provenzano et al. 2019[5]
To understand the neurological risks of intrathecal gadolinium, it is critical to understand gadolinium’s chemistry, pharmacology, and proposed mechanisms of toxicity. Gadolinium is toxic in its free, ionic state (Gd3+).[6] In an effort to limit the toxicity of free gadolinium, the gadolinium ion is bound to a chelating ligand.[7] These chelating ligands are characterized based on their shape as either linear or macrocyclic. GBCAs can be further classified as ionic or nonionic depending on if the compound is electrically neutral (nonionic) or charged (ionic). Macrocyclic GBCAs form a tight “cage” around the gadolinium ion, making them more stable (less likely to release the gadolinium ion) than linear GBCAs. Ionic GBCAs have a higher thermodynamic stability constant (stronger bond between the gadolinium ion and chelating ligand) than nonionic GBCAs. [1] There are nine different brands of GBCAs: gadofosveset trisodium, gadoxetate disodium, gadopentetate dimeglumine, and gadobenate dimeglumine (linear ionic); gadodiamide and gadoversetamide (linear nonionic); gadoterate meglumine (macrocyclic ionic); gadobutrol and gadoteridol (macrocyclic nonionic) (Table 2). The prescribing information from eight out of the nine brands mention the risk of serious neurological adverse events after GBCA administration. Of these, gadodiamide is the only brand that mentions the neurological risks of intrathecal administration, and its prescribing information specifically states that gadodiamide is not to be used intrathecally.
Table 2: Prescribing information of different brands of GBCAΦ
Generic name | Trade name | Molar concentration of gadolinium (mol/L) | Documents the risk of serious nervous system-related complications? |
Gadofosveset trisodium | Ablavar | 0.25 | No |
Gadoterate meglumine | Dotarem | 0.5 | Yes |
Gadoxetate disodium | Eovist | 0.25 | Yes |
Gadobutrol | Gadavist | 1 | Yes |
Gadopentetate dimeglumine | Magnevist | 0.5 | Yes |
Gadobenate dimeglumine | MultiHance | 0.5 | Yes |
Gadodiamide | Omniscan | 0.5 | Yes* |
Gadoversetamide | Optimark | 0.5 | Yes |
Gadoteridol | ProHance | 0.5 | Yes |
*Specifically indicated the risk of neurotoxicity due to intrathecal administration
Source: 2012 Hao and prescribing info[1]
Φ Adapted from Provenzano et al. 2019[5]
Transmetalation is the major method of in vivo dechelation. Dechelation of the gadolinium ion occurs when endogenous ions (namely, Cu2+, Fe3+, Zn2+, and Ca2+) compete for gadolinium’s chelating ligand. [1,8] Research has mainly focused on in vivo gadolinium transmetalation with intravenous use, not intrathecal use. Therefore the influence of transmetalation on intrathecal gadolinium neurotoxicity is unclear. [1]
There have been several proposed mechanisms for acute gadolinium-induced neurotoxicity. Feng et al.[9] found that gadolinium exposure quickly led to disruption of mitochondrial function in rat cortical neurons. Mitochondrial dysfunction resulted in an accumulation of reactive oxygen species, which inhibited cellular function and induced apoptosis. Xia et al.[10] also studied gadolinium neurotoxicity in rat cortical neurons and demonstrated that oxidative stress from mitochondrial disruption led to strain in the endoplasmic reticulum. Alterations in the endoplasmic reticulum resulted in the activation of the apoptotic pathway. In another study, Feng et al.[11] observed dramatic extracellular calcium ion influx in astrocytes after gadolinium exposure. Elevated calcium levels may play an important role in apoptosis. Gadolinium disrupts multiple calcium-dependent pathways since its ionic radius is similar in size to calcium, allowing it to compete with calcium in vivo. Gadolinium has been shown to block voltage-gated calcium channels, inhibit calcium-activated enzymes, and function as an agonist in calcium receptors. These qualities give gadolinium a wide range of detrimental effects, from mitochondrial disruption and oxidative stress, to inhibition of muscle contraction and nerve signal transmission.[8]
Acute intrathecal gadolinium-induced neurotoxicity has been demonstrated in multiple animal models. Ray et al.[12] injected gadopentetate dimeglumine into the lateral ventricles of rats. Dose-dependent neurotoxicity was seen with symptoms beginning at gadolinium concentrations of 5 μmol/g brain. Affected rats demonstrated behavioral changes and focal lesions in the thalamus, brain stem, and spinal cord. No evidence of neurological decline was observed in rats that received gadolinium concentrations of 2.5 or 3.3 μmol/g brain. In a second study, Ray et al. [13] performed a similar experiment, this time including the GBCA gadodiamide. Once again, dose-dependent neurotoxicity was seen, with morphological changes beginning at 1.25 μmol/g brain and behavioral changes beginning at 2.5 μmol/g brain. Toney et al. found that 2.5 μmol/g brain intrathecal gadopentetate dimeglumine caused no significant signs of neurotoxicity in rats. [14] However, documented histological changes were seen including gliosis and inflammation.
Numerous documented cases of intrathecal GBCA acute neurotoxicity have been reported in humans with outcomes ranging from transient signs and symptoms with no long-term sequelae to death (Table 1). Neurotoxic effects resulted from both intentional and unintentional intrathecal delivery of GBCAs. Neurotoxic intrathecal gadolinium concentrations ranged from 1.07 μmol/g brain to 7 μmol/g brain (Table 1). In human case series and reports using lower concentrations (i.e. 0.07 to 0.36 μmol/g brain) of intrathecal gadolinium, no signs of acute neurotoxicity were seen (Figure 1). [15-21] Alarmingly, dose ranges found to be relatively safe in animal models (e.g. rats) have been demonstrated to be neurotoxic and even fatal in humans. For example, doses as high as 3.33 μmol/g brain have caused no neurotoxic effects in rats, but doses as low as 2.3 μmol/g brain have been fatal in humans. [5,12] Notably, doses lower than 2.3 μmol/g brain (1.07, 1.43, and 2.14 μmol/g brain) have been neurotoxic in humans, but not fatal (Table 1). The disparity between animal and human neurotoxicity at similar concentrations undermines the efficacy of directly comparing animal and human responses to intrathecal gadolinium. This highlights the need for further research into the specific mechanism and risk factors for human gadolinium-induced neurotoxicity.
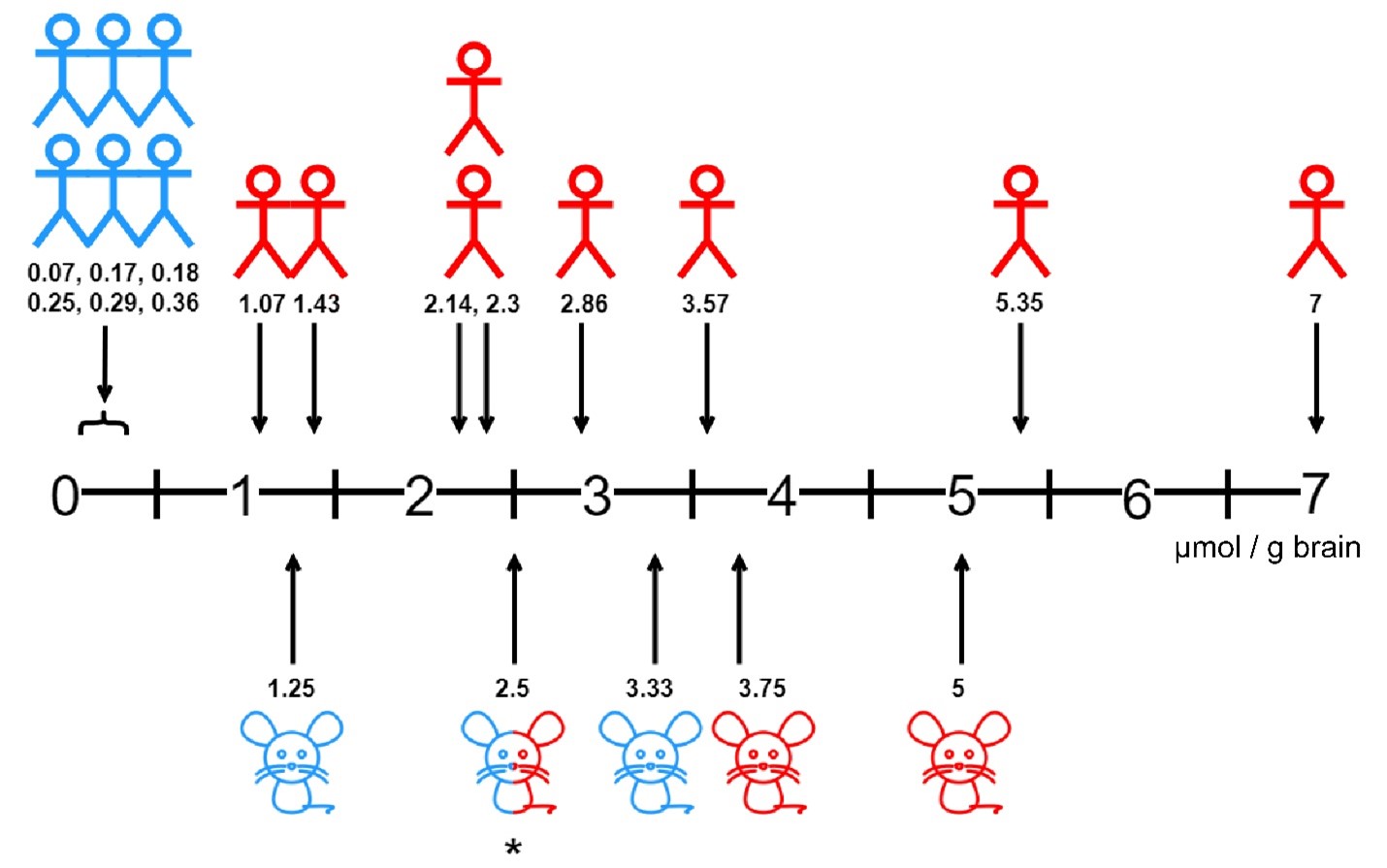 Figure 1: Neurotoxic gadolinium concentrations in human and rat studies.
Figure 1: Neurotoxic gadolinium concentrations in human and rat studies.
Numerical values indicate gadolinium concentration in µmol per gram of brain. Human drawings indicate human studies, rat drawings indicate rat studies. Concentrations that did not induce neurological complications are shaded blue,
while concentrations that induced neurological complications are shaded red. *A gadolinium concentration of 2.5 µmol/g brain did not induce neurological complications in 2 publications[12,14] but did induce neurological complications
in 1 publication when the gadolinium was not injected over an extended period of time.[13]
Specific volumes and dose limits for GBCAs have been suggested as viable visualization alternatives in interventional pain procedures when there is the possibility of inadvertent or deliberate intrathecal administration. [3,22] First, we will remind the reader that the administration of a GBCA via routes other than intravenous is an off label use and that significant nervous system-related warnings are listed in the prescribing information. [5] Second, specific formulations of GBCAs have differing chelating agents and concentrations of gadolinium and thus the toxicity profile of one type is not directly applicable to another. When looking at the limited published clinical reports of acute GBCA neurotoxicity to date, physicians may desire to stay below a gadolinium dose of 1 μmol/g brain (ie, theoretical number based on case reports suggesting neurological risks). Based on a theoretical dose limit of 1 μmol/g brain, restricted GBCA volumes based on the GBCA’s gadolinium molar concentration would be feasible. GBCA volume limits would range between 1.4 mL (1 mol/L of gadolinium) to 5.6 ml (0.25 mol/L of gadolinium). The relative radiographic conspicuity of a GBCA is directly correlated to the gadolinium concentration of the agent selected.[23] Gadolinium-based contrast agents that have a higher gadolinium concentration are more visible under fluoroscopic imaging, but lower volumes would have to be used to stay below the theoretical neurotoxic intrathecal administration level.
Intrathecal gadolinium poses a serious risk of neurotoxicity, even at small doses. GBCAs are not FDA approved for use in the intrathecal or epidural spaces. Furthermore, if GBCAs are being considered as an alternative to iodine-based contrast agents for visualization in interventional pain procedures, one must remember that this is an off-label use with significant risk of acute neurotoxicity when intrathecal administration occurs intentionally or unintentionally. Since different types of GBCA have different chelating agents and concentrations of gadolinium, the toxicity profile of one type is not directly applicable to another. Extensive research is required to better understand the risk of GBCA neurotoxicity. Until further basic and clinical research is performed, GBCAs should not be considered a safe and viable alternative to iodine-based contrast in neuraxial interventional pain procedures for which there is the risk of intrathecal administration.
References
- Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M. MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging. 2012;36(5):1060-71.
- Safriel Y, Ali M, Hayt M, Ang R. Gadolinium use in spine procedures for patients with allergy to iodinated contrast-experience of 127 procedures. AJNR Am J Neuroradiol. 2006;27(6):1194-7.
- Hagedorn JM, Bendel MA, Moeschler SM, Lamer TJ, Pope JE, Deer TR. Intrathecal gadolinium use for the chronic pain physician. Neuromodulation. 2019;22(7):769-74.
- Ramalho J, Ramalho M, Jay M, Burke LM, Semelka RC. Gadolinium toxicity and treatment. Magnetic resonance imaging. 2016;34(10):1394-8.
- Provenzano DA, Pellis Z, DeRiggi L. Fatal gadolinium-induced encephalopathy following accidental intrathecal administration: a case report and a comprehensive evidence-based review. Reg Anesth Pain Med. 2019;44:721–729. doi:10.1136/rapm-2019-100422.
- Sherry AD, Caravan P, Lenkinski RE. Primer on gadolinium chemistry. J Magn Reson Imaging. 2009;30(6):1240-8.
- Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99(9):2293-23c52.
- Ramalho J, Semelka RC, Ramalho M, Nunes RH, AlObaidy M, Castillo M. Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol. 2016;37(7):1192-8.
- Feng X, Xia Q, Yuan L, Yang X, Wang K. Impaired mitochondrial function and oxidative stress in rat cortical neurons: implications for gadolinium-induced neurotoxicity. Neurotoxicology. 2010;31(4):391-8.
- Xia Q, Feng X, Huang H, Du L, Yang X, Wang K. Gadolinium-induced oxidative stress triggers endoplasmic reticulum stress in rat cortical neurons. J Neurochemistry. 2011;117(1):38-47.
- Feng XD, Xia Q, Yuan L, Huang HF, Yang XD, Wang K. Gadolinium triggers unfolded protein responses (UPRs) in primary cultured rat cortical astrocytes via promotion of an influx of extracellular Ca2+. Cell Biol Toxicol. 2011;27(1):1-12.
- Ray DE, Cavanagh JB, Nolan CC, Williams SC. Neurotoxic effects of gadopentetate dimeglumine: behavioral disturbance and morphology after intracerebroventricular injection in rats. AJNR Am J Neuroradiol. 1996;17(2):365-73.
- Ray DE, Holton JL, Nolan CC, Cavanagh JB, Harpur ES. Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. AJNR Am J Neuroradiol. 1998;19(8):1455-62.
- Toney GM, Chavez HA, Ibarra R, Jinkins JR. Acute and subacute physiological and histological studies of the central nervous system after intrathecal gadolinium injection in the anesthetized rat. Invest Radiol. 2001;36(1):33-40.
- Algin O, Turkbey B. Intrathecal gadolinium-enhanced MR cisternography: a comprehensive review. AJNR Am J Neuroradiol. 2013;34(1):14-22.
- Akbar JJ, Luetmer PH, Schwartz KM, Hunt CH, Diehn FE, Eckel LJ. The role of MR myelography with intrathecal gadolinium in localization of spinal CSF leaks in patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2012;33(3):535-40.
- Dillon WP. Intrathecal gadolinium: its time has come? AJNR Am J Neuroradiol. 2008;29(1):3-4.
- Albayram S, Kilic F, Ozer H, Baghaki S, Kocer N, Islak C. Gadolinium-enhanced MR cisternography to evaluate dural leaks in intracranial hypotension syndrome. AJNR Am J Neuroradiol. 2008;29(1):116-21.
- Tali ET, Ercan N, Kaymaz M, Pasaoglu A, Jinkins JR. Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced MR cisternography used to determine potential communication between the cerebrospinal fluid pathways and intracranial arachnoid cysts. Neuroradiol. 2004;46(9):744-54.
- Tali ET, Ercan N, Krumina G, et al. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol. 2002;37(3):152-9.
- Zeng Q, Xiong L, Jinkins JR, Fan Z, Liu Z. Intrathecal gadolinium-enhanced MR myelography and cisternography: a pilot study in human patients. AJR American journal of roentgenology. 1999;173(4):1109-15.
- Popescu A, Patel J, McCormick ZL, et al. Fact finders for patient safety: are gadolinium-based contrast media safe alternatives to iodinated contrast agents for the safe performance of spinal injection procedures? Pain Med. 2018;19(10):2089-90.
- Maus TP, Schueler BA, Magnuson DJ, Magnuson D. Relative conspicuity of gadolinium-based contrast agents in interventional pain procedures. Pain Med. 2017;18(4):651-4.
- Arlt S, Cepek L, Rustenbeck HH, Prange H, Reimers CD. Gadolinium encephalopathy due to accidental intrathecal administration of gadopentetate dimeglumine. J Neurology. 2007;254(6):810-2.
- Li L, Gao FQ, Zhang B, Luo BN, Yang ZY, Zhao J. Overdosage of intrathecal gadolinium and neurological response. Clin Radiol. 2008;63(9):1063-8.
- Kapoor R, Liu J, Devasenapathy A, Gordin V. Gadolinium encephalopathy after intrathecal gadolinium injection. Pain Physician. 2010;13(5):E321-6.
- Park KW, Im SB, Kim BT, Hwang SC, Park JS, Shin WH. Neurotoxic manifestations of an overdose intrathecal injection of gadopentetate dimeglumine. J Korean Med Sci. 2010;25(3):505-8.
- Nayak NB, Huang JC, Hathout GM, Shaba W, El-Saden SM. Complex imaging features of accidental cerebral intraventricular gadolinium administration. J Neurosurg. 2013;118(5):1130-4.
- Reeves C, Galang E, Padalia R, Tran N, Padalia D. Intrathecal injection of gadobutrol: a tale of caution. J Pain Palliat Care Pharmacother. 2017;31(2):139-43.
Leave a commentOrder by
Newest on top Oldest on top