How I Do It: Minimally Invasive Lumbar Decompression
Cite as: Malik T, Amiri Y. How I do it: minimally invasive lumbar decompression. ASRA News 2020;45. https://doi.org/10.52211/asra110120.064
Minimally invasive lumbar decompression (Mild®, Vertos Medical) is an image-guided approach used in the treatment of symptomatic lumbar central spinal stenosis. The Mild kit received 501k (FDA marketing clearance) in 2006. The Centers for Medicare and Medicaid Services (CMS) restored coverage for this procedure in 2017 after having previously withheld reimbursement secondary to safety concerns.
The Mild procedure is a good option for patients who are not responsive to conservative or injection therapy and are either not good surgical candidates or do not want open surgical decompression.
Lumbar spinal stenosis (LSS) is a degenerative disease of the elderly spine and is the most common indication for spinal surgery in patients age 65 and above. Data from the National Ambulatory Medical Care Survey and the National Spine Network estimate the prevalence of LSS at 13-14% in patients with low-back problems who visit a specialist and 3-4% in the population who visit a general physician.[1] According to the Framingham population study, spinal stenosis is found, per radiological criteria, in 19-47% of Americans over the age of 60.[2] The prevalence is bound to increase due to demographic trends in the U.S. population.[3] LSS is a clinical diagnosis. The etiology is usually multifactorial, and causative factors include a variable combination of ligament flavum hypertrophy, anterolisthesis, disc protrusion, or facet joint hypertrophy.[4] However, flavum hypertrophy tends to contribute the most to spinal stenosis.[5] LSS is generally managed conservatively with physical therapy, NSAIDs, or injection therapy (caudal or lumbar epidural steroid injection). The effectiveness of these modalities is variable.[6] However, less-invasive options should be tried prior to offering surgery. Surgery is portrayed as a definite treatment option but is not always effective, and complication rates as high as 40% have been reported.[7] Patients often need another surgery (reoperation rate of 23% in one series) and may still have significant residual discomfort.[8] Not every patient is a good surgical candidate due to coexisting medical issues. The Mild procedure is a good option for patients who are not responsive to conservative or injection therapy and are either not good surgical candidates or do not want open surgical decompression.
Pathophysiology of Lumbar Spinal Stenosis
The exact mechanism of neurogenic claudication is unclear. The accepted etiological theory is either microvascular ischemia or venous obstruction leading to inadequate oxygenation and or metabolite accumulation.[9,10] There also may be a component of direct physical compression, which explains worsening of symptoms with various positions.[11] The evidence for inflammation is not strong.
Indication
The Mild procedure is indicated for patients with symptomatic central LSS due to ligamentum flavum hypertrophy. It is not meant for patients with symptoms due to lateral recess spinal stenosis. It is also not indicated if stenosis is due to any cause other than ligamentum flavum hypertrophy (eg, anterolisthesis or disc protrusion) (Figure 1).
Indications
| Contraindications
|
Figure 1: Indications and contraindications for the Mild procedure
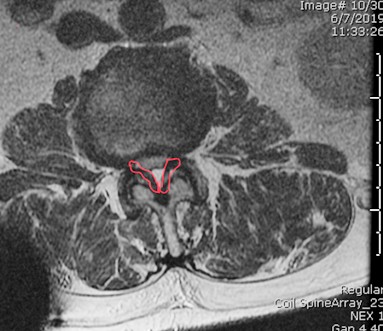
Figure 2: Thickened ligamentum flavum outlined in red on axial view MRI lumbar spin
Patient Selection
Patient selection is the most important part of the Mild procedure. The patient should have symptomatic central LSS from ligamentum flavum hypertrophy (Figure 2). The procedure is planned when the patient has neurogenic claudication in the presence of radiologically proven ligamentum flavum hypertrophy. The cardinal symptoms of neurogenic claudication are pain, numbness, weakness, and tingling in the low back, buttocks, and legs, initiated by standing, walking, or lumbar extension and relieved by sitting or forward flexion. The symptoms do not follow a dermatomal pattern (a hallmark of foraminal or lateral recess stenosis), but the two distinct etiologies can coexist. The symptoms tend to be symmetrical, usually above the knee. This proximal distribution helps to symptomatically differentiate lumbar stenosis from vascular claudication.[12] Physical examination is unremarkable, and the presence of sensory or motor deficits should prompt surgical evaluation. Imaging is required for the definite diagnosis of LSS.[13] A systemic review found CT or MRI equally accurate in the diagnosis of LSS.[14] MRI is preferred as ligament hypertrophy can be easily measured. There are a number of radiological criteria used to diagnose spinal stenosis, but anterior-posterior spinal canal diameter of <10 mm or an area of < 70mm2 is generally considered diagnostic.[15] The criteria used in various Mild trials were patients with neurogenic claudication for at least three months, ligamentum flavum thickness of > 2.5 mm, anterolisthesis of 5mm or less, and an absence of spinal instability.
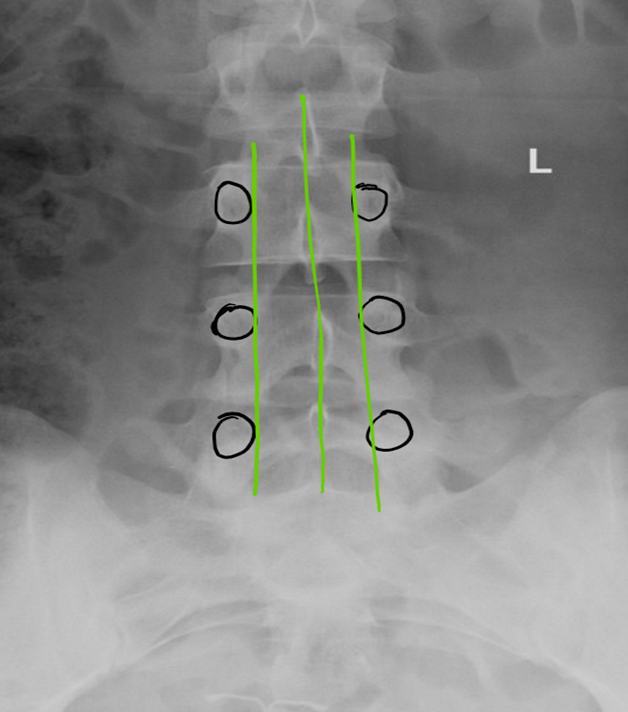
Figure 3: Three green lines drawn define the working area. Black circles outline the pedicles
Procedure
Anesthesia: The procedure is usually done with mild to moderate sedation. Deep sedation or general anesthesia should be avoided unless performed with nerve monitoring.
Positioning: Optimally position the patient in the prone position. In other words, flatten or minimize lumbar lordosis, with no pressure on the belly or chest for ease of breathing for the patient, and enable the patient to be comfortable enough to lay on the table for an hour or so.
Antimicrobial Actions: 1-2 gm cephazolin or another appropriate antibiotic is given before the start of the procedure. The target area is scrubbed with 2-3% chlorhexidine/70% isopropyl alcohol solution and 3 minutes are allowed for it to dry. Full surgical drapes are used, and the target skin area is covered with an antimicrobial incise drape (eg, IobanTM).
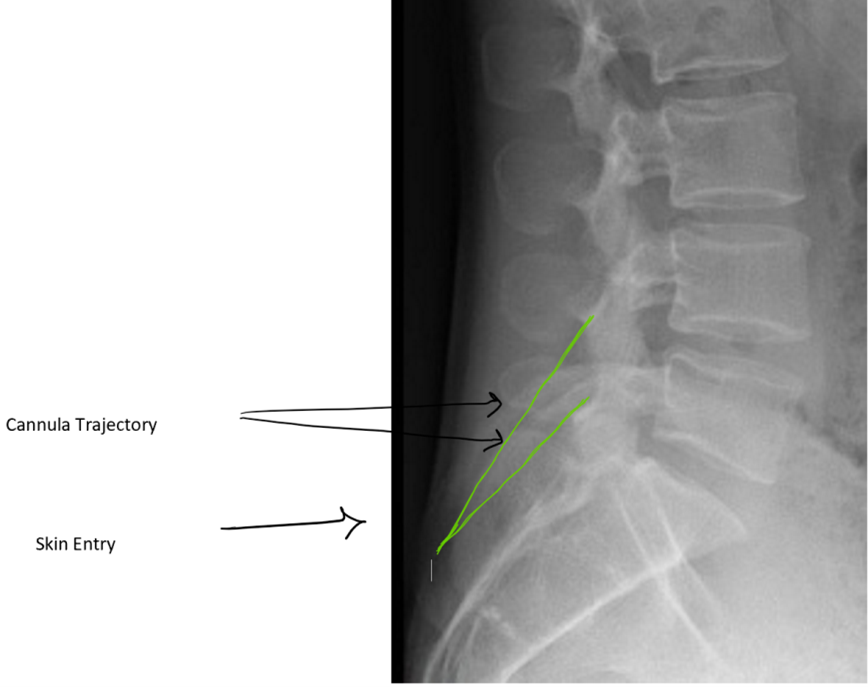
Figure 4: Planning trajectory using lateral view: Green lines demonstrate working cannula trajectory (double arrows point to cannula). Skin entry (demonstrated by single black arrow) is at the lower portion of the lamina
Procedure: It is imperative to review patient anatomy by reviewing available radiological images prior to starting the procedure. Confirm the presence of pathology at the target vertebral level, evaluate the dimension of interlaminar space, identify any bony overgrowth, and, most importantly, evaluate and plan the trajectory of trocar insertion. An anterior-posterior fluoroscopic view of the lumbar spine is obtained, with the spinous process midway between the pedicles. Three straight line are drawn: one in the middle (over the spinous processes), and the other two connecting the medial side of the pedicles on each side (Figure 3).
The procedure starts with obtaining epidural space access. An epidural needle should be placed in the most cephalad part of the interlaminar space with the intent to obtain ipsilateral dye spread. The optimal spot on the skin for inserting the working cannula is chosen after reviewing the radiological images. It is usually 1½ to 2 vertebral levels below the target space, in the paramedian plane between the two lines initially drawn on the patient at the start of the case. After cutaneous analgesia is provided with a 25 g needle, the cannula tract is further anesthetized with a 22 g 3.5 inch spinal needle to the lower lamina, including the laminar periosteum. After a skin stab, a 6 g working cannula via use of the trochar is inserted to the lower lamina. Lateral fluoroscopic guidance is used to ensure that it is not placed too deep (which will result in a large dural tear) (Figures 4,5).
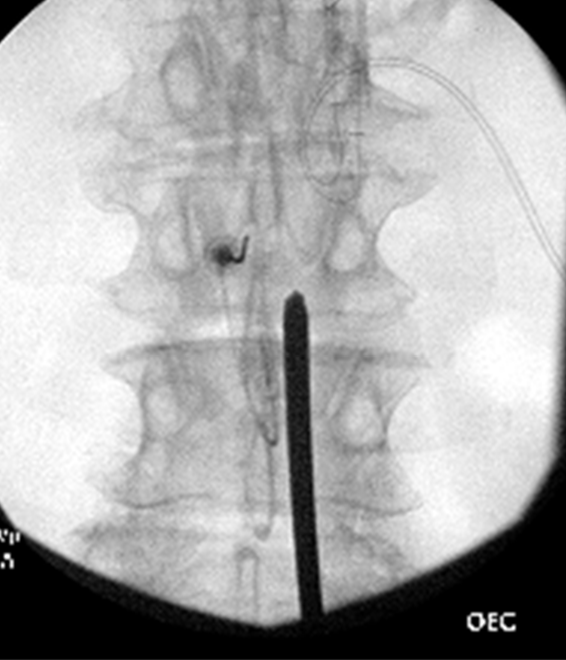
Figure 5: Anterior-posterior view of working cannula placed at the lower lamin
Next, a contralateral oblique view is obtained. The optimal view is one that allows laminar outlines to appear the thickest and the crispest. This angle is usually around 40-45 degrees contralateral oblique. Cephalocaudal tilting may be required to obtain the optimal working view. The intention is to get the x-ray beam perfectly parallel to the ipsilateral lamina. The configuration of epidurogram seen in this view confirms the location of the stenosis as well as serves as a safety check and constant reminder of the plane that should not be violated. Therefore, it is important to maintain the epidurogram by frequently injecting dye throughout the procedure. The improvement in the contour of the epidurogram is one of the endpoints of this procedure. The epidurogram represents the plane, which should not be violated with any instrument during the procedure.
After the working cannula is in place, a portal stabilizer device is used to stabilize the cannula. The device grips the cannula, thus preventing it from inadvertently advancing during the procedure. The trocar is removed, and another piece from the kit, the depth guide device, is placed over it. The depth guide limits the risk of subsequent instruments unintentionally being pushed too far in through the cannula. A bone ronguer is advanced through the portal under fluoroscopic guidance. The space between the laminae is opened up by snipping away bone, first from the lower edge of the upper lamina and then by flipping the ronguer and snipping from the upper edge of the lower lamina (Figure 6). This process also loosens the ligamentum flavum attachment from the lower lamina making it easier to sculpt. The interlaminar space is opened up by repeating these steps more medially and laterally, thus creating a good opening. Fluoroscopic guidance should be used at all times. The lines drawn at the start of the procedure (Figure 2) are helpful to avoid angling the ronguer too far medially or laterally. The trigger of the bone ronguer should be pulled slowly in case one needs to stop the bite at any time. It is important to be patient and judiciously remove small amounts of bone with any bite. The depth guide device may need adjustments to gain access to the deeper part of the laminae. Once enough space has been created, the tissue sculptor is inserted through the portal. The tissue sculptor end is shaped like an ice cream scooper and is specifically designed with the cutting edge on the inner lip. It only scrapes tissue when employing an upward scooping movement (Figure 7). The stroke starts from the lower lamina and ends at the upper lamina, at which point the trigger of the tissue sculptor is pulled, which resects the ligament flavum and collects the resected tissue. All tissue bites should be performed with live fluoroscopy in the contralateral oblique view. The movement is repeated a few times, and then the sculptor is retracted and any resected tissue is removed by pushing it out of the sculptor with the thumb trigger. The procedure is repeated until enough tissue has been removed. Adequate tissue removal is confirmed via visualization of straightening and thickening of the epidurogram outline as an indication that the spinal space has opened up. Additional ligament may need to be removed either medially and/or laterally and should be guided by MRI imaging.
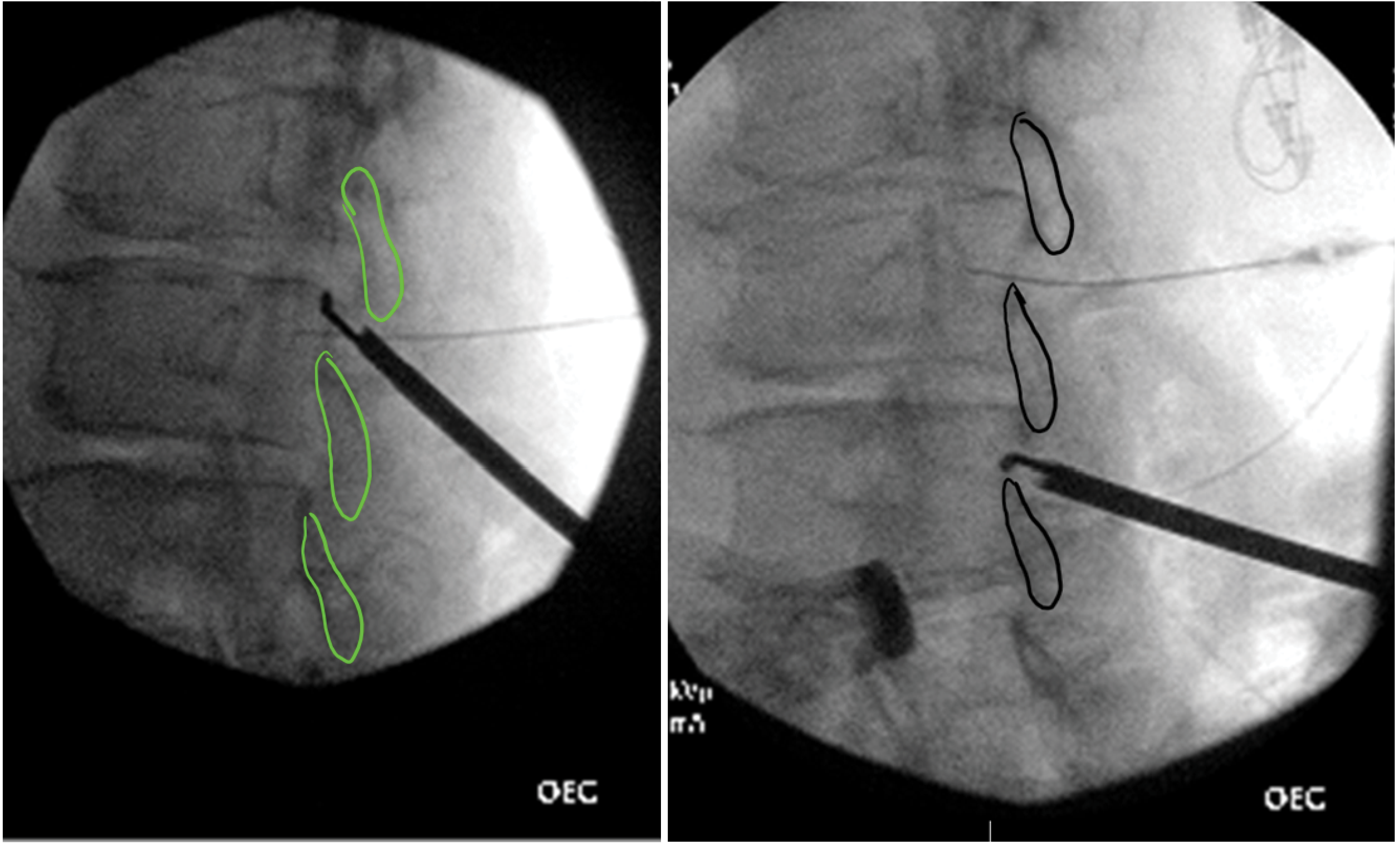
Figure 6: Bone ronguer in (left) contralateral oblique view in cephalad position and (right) contralateral oblique view in caudad position.
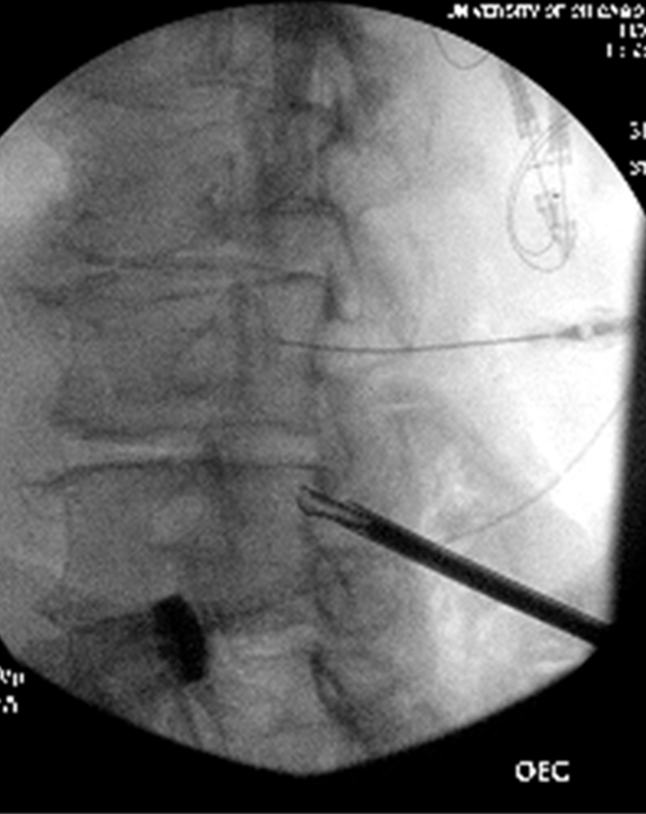
Figure 7: Tissue sculptor in contralateral oblique view
The procedure removes only the dorsal part of the ligament flavum, leaving the ventral part intact. The amount of tissue removed is not related to the extent of pain relief obtained, and it is not necessary to measure the volume of tissue removed. Once the epidural space has opened up enough, as seen on the epidurogram, the working cannula is removed. The epidural needle is removed partially and redirected to obtain epidural space access in the contralateral side. The working cannula is placed on the other side and the whole procedure is repeated. Once the treatment of spinal stenosis at one level is complete, the procedure may be repeated at an additional interlaminar level. The procedure may be performed at two adjacent spinal levels via use of a single skin puncture if the patient’s physique is amenable. This dual-level approach requires performing the procedure on the ipsilateral side at two interlaminar spaces and then removing the portal prior to insertion on the contralateral side. Once the procedure endpoint has been achieved, the cannula is removed and the skin is closed with skin glue and steri-strips. Suturing is not generally required.
Post-Procedure Care
There is no need for post-procedure antibiotic coverage. The patient is observed in the recovery area until sedation wears off. The patient can ambulate freely once at home. The procedural pain should ease up in few days. A brief neurological examination is performed before the patient is discharged home. A follow-up visit is scheduled in 2 weeks to evaluate the extent of symptom relief. At times, optimal functional improvement requires a course of physical therapy for muscle strengthening and conditioning. Therefore, a final assessment of patient improvement may not be feasible until a few months post-procedure.
Complications: There is always a risk of infection, bleeding, or nerve damage. No such complication has been reported in published studies. There is risk of a dural tear which would be large, but also has not been reported.
Procedure Effectiveness
A number of observational and few randomized studies have shown that the procedure provides durable relief and meaningful improvements in functional status in properly selected patients. The 2-year follow up of patients enrolled in the MiDAS ENCORE trial showed a mean improvement in visual analog scale score of 3.6 points and a mean improvement in Oswestry Disability Index of 22.7 points.[16] A systematic review found that although the procedure seems to be relatively safe, the current evidence on Mild for the treatment of symptomatic lumbar spinal stenosis is of low quality and suffers from a risk of bias.[17] In addition, no post-procedure imaging study has been completed that has demonstrably correlated symptomatic improvements with improvements in the extent of central spinal stenosis. Though Mild has demonstrated efficacy compared to lumbar epidural steroid injections, the procedure has not been compared head-to-head with surgery.[19,20]
Clinical Pearls
- Ensure symptoms are related to central spinal stenosis.
- Insert the epidural needle high in the interlaminar space, out of way of other instruments.
- Ensure an ipsilateral epidurogram for each side.
- Ensure adequate opening of the interlaminar space using a bone ronguer.
- Remove enough tissue to improve the contour of the epidurogram.
References
- Agency for Healthcare Research and Quality. Treatment of degenerative lumbar spinal stenosis. Summary, Evidence Report/Technology Assessment Number 32. AHRQ Publication No. 01-E047, March 2001. Rockville, MD. Available at: https://www.ncbi.nlm.nih.gov/books/NBK11855/. Accessed October 15, 2020.
- Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:54550.
- The United States Census. United States population projections: 2000-2050. 2009. Available at: https://www.census.gov/library/working-papers/2009/demo/us-pop-proj-2000-2050.html. Accessed October 15, 2020.
- Kawaguchi Y, Kanamori M, Ishihara H, et al. Clinical and radiographic results of expansive lumbar laminoplasty in patients with spinal stenosis. J Bone Joint Surg Am. 2004;86:1698–703.
- Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679–86.
- Ammendolia C, Stuber KJ, Rok E, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev. 2013;8;CD01071.
- Benz RJ, Ibrahim ZG, Afshar P, et al. Predicting complications in elderly patients undergoing lumbar decompression. Clin Orthop Relat Res. 2001;384:116–21.
- Katz JN, Lipson SJ, Chang LC, et al. Seven- to 10-year outcome of decompressive surgery for degenerative lumbar spinal stenosis. 1996;21:92–9.
- Watanabe R, Parke WW. Vascular and neural pathology of lumbosacral spinal stenosis. J Neurosurg. 1986;64:64-70.
- Ooi Y, Mita F, Satoh Y. Myeloscopic study on lumbar spinal canal stenosis with special reference to intermittent claudication. 1990;15:5449.
- Binder DK, Schmidt MH, Weinstein PR. Lumbar spinal stenosis. Semin Neurol. 2002;22:157-66
- Nadeau M, Rosas-Arellano M, Gurr K, et al. The reliability of differentiating neurogenic claudication from vascular claudication based on symptomatic presentation. Can J Surg. 2013;56:372-7.
- De Schepper EIT, Overdevest GM, Suri P, et al. Diagnosis of lumbar spinal stenosis: an updated systematic review of the accuracy of diagnostic tests. 2013;38:E469-8.
- De Graaf I, Prak A, Bierma-Zeinstra S, et al. Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. 2006;31:1168-76
- Steurer J, Roner S, Gnannt R, et al. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175.
- Staats PS, Benyamin RM, MiDAS ENCORE Investigators. MiDAS ENCORE: randomized controlled clinical trial report of 6-month results. Pain Physician. 2016;19(2):25–38.
- Kreiner DS, MacVicar J, Duszynski B, et al. The mild procedure: a systematic review of the current literature. Pain Med Malden Mass. 2014;15:196-20
- Kim YU, Park JY, Kim DH, et al. The role of the ligamentum flavum area as a morphological parameter of lumbar central spinal stenosis. Pain Physician. 2017;20(3):E419–E424.
- Brown LL. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 2012;12(5):333–41.
- Benyamin RM, Staats PS, MiDAS Encore I. Mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE randomized controlled trial. Pain Physician. 2016;19(4):229–42.
Leave a commentOrder by
Newest on top Oldest on top