Restorative Neurostimulation for Intractable Mechanical Chronic Low Back Pain
Cite as: Gilligan C. Restorative neurostimulation for intractable mechanical chronic low back pain. ASRA News 2021;46. https://doi.org/10.52211/asra080121.053.
Symptoms of intractable mechanical chronic low back pain (CLBP) are frequently linked to impaired neuromuscular control and degeneration of the multifidus muscle, the primary local stabilizer of the lumbar spine.1–6 Functional instability causes ongoing, mechanically induced pain in the joints and surrounding structures. Given that surgery is not indicated for most patients and other available treatment options have limited effectiveness or durability, effective pain management is an important clinical need that has long remained unaddressed.
A novel implantable restorative neurostimulation system to relieve pain and improve function by restoring multifidus motor control (ReActiv8®, Mainstay Medical) was approved in 2020 by the U.S. Food and Drug Administration for use in the United States. The ReActiv8-B pivotal study, a multicenter, prospective, randomized, sham-controlled trial, demonstrated the treatment’s safety, effectiveness and durability (NCT02577354; publication under review).
Etiology and Pathophysiology of CLBP
Nonsurgical treatments for mechanical CLBP such as physical therapy, nonopioid medications, opioid analgesics, injections, and rhizotomy may provide transient relief but rarely offer long-term improvement.7–9 Although spinal cord stimulation is frequently used for CLBP with radiculopathy into the lower extremities, particularly following spine surgery, expert consensus is that it is not appropriate for patients with predominantly nociceptive mechanical CLBP.10 If conservative therapy fails to provide pain relief, ongoing management for mechanical CLBP usually consists of coping mechanisms and pain medications, which are often increased during episodic flares.
Impaired Neuromuscular Control of Lumbar Spine
The multifidus muscle is innervated by the medial branch of the dorsal ramus, a mixed nerve that also innervates intramuscular proprioceptors and zygoapophysial (facet) joint nociceptors. Following an acute initial injury of the joint structure, the multifidus’s neuromuscular control may be disrupted by a presynaptic, ongoing reflex inhibition of musculature surrounding the joint, a natural response designed to protect the joint from further damage. If ongoing noxious stimuli constantly retriggers arthrogenic inhibition, patients may eventually develop muscle atrophy and weakness.11 Figure 1 depicts images of mild (1A), moderate (1B), and severe (2C) fatty infiltration of the multifidus muscle representing signs of atrophy.
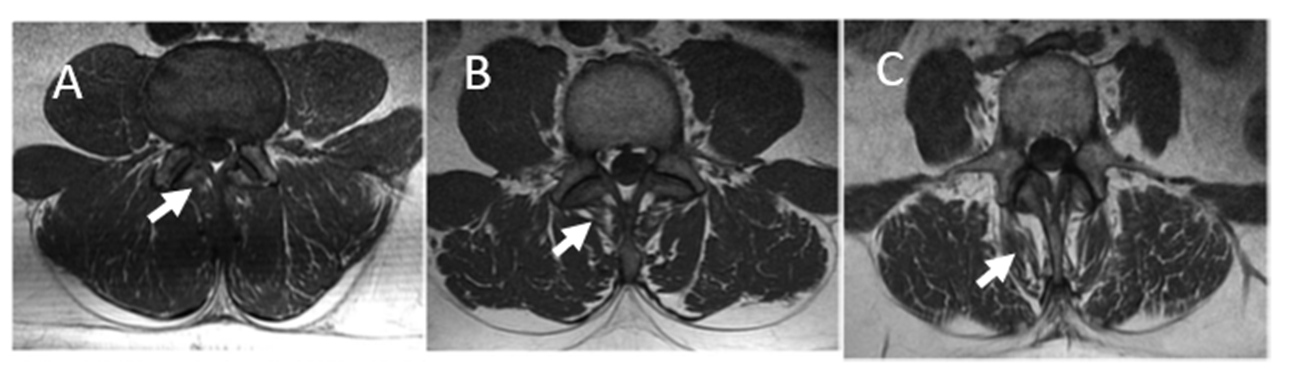
Figure 1. T1-weighted magnetic resonance images of lumbar spine at L3 showing 1A (mild; 10%), 1B (moderate; 10%–50%) and 1C (severe; > 50%) fatty infiltration of the lumbar multifidus muscle
Images are from subjects enrolled in the ReActiv8-B clinical trial, used with permission.
Impaired multifidus neuromuscular control is associated with changes in the muscle’s cortical representation and an inability to exert voluntary control.12 Targeted exercises enable some patients to override the motor control system inhibition and experience consequent improvement in back pain.13 The proposed mechanism of action that has been studied is the restoration of neuromuscular control of the multifidus via repetitive isolated multifidus contraction, thereby leading to improved segmental spine stability and reduced low back pain. Unfortunately, targeted multifidus neuromuscular control exercises are difficult to perform and teach, and many patients are simply unable to voluntarily contract a muscle group not normally amenable to voluntary control. In addition, back pain may induce arthrogenic inhibition of spine stabilizing muscles, also inhibiting any voluntary contraction of the multifidus.
Restorative Neurostimulation for Mechanical CLBP
The ReActiv8 restorative neurostimulation system invokes the same restorative mechanism by delivering electrical stimulation to the bilateral L2 medial branches of the dorsal rami to elicit episodic contractions of the lumbar multifidus muscle and hence override the underlying inhibition. Two studies in patients with disabling mechanical CLBP showed that restorative neurostimulation treatment effects accrue over time and that improvements in pain and function are durable and clinically meaningful.14,15
The system’s overall safety profile compares favorably to published data from studies of other implantable neurostimulation systems. Because the stimulation target is outside of the spinal canal, risk of spinal cord injury during lead placement is negligible. The risk of lead migration—the most common adverse event reported in neurostimulation trials—has been effectively mitigated with a lead design incorporating flexible distal tines.
Patient Selection
Restorative neurostimulation was developed for and evaluated in patients experiencing disabling, intractable, mechanical CLBP that results in pain at least half of the days in the past 12 months and who have evidence of impaired multifidus neuromuscular control. Patients experiencing radicular CLBP, have indications for spine surgery, or had prior spine surgery have thus far been excluded from clinical trials.
Device, Implant Procedure, and Therapy Process
The system consists of an implantable pulse generator (IPG), two stimulation leads (Figure 2), a radiofrequency telemetry programmer, and patient-controlled therapy session activator.
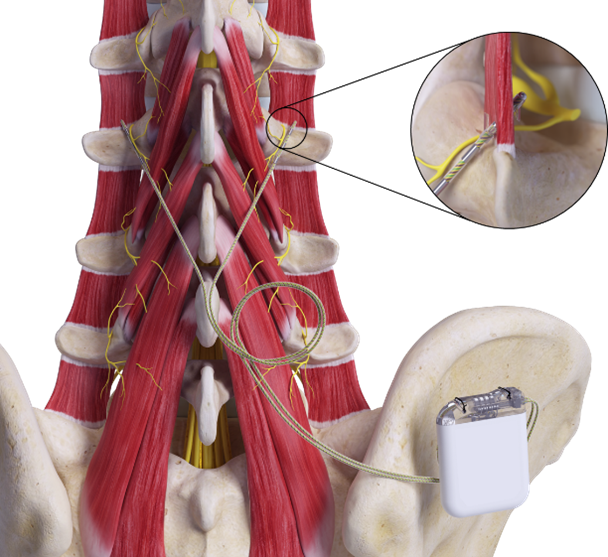
Figure 2. Implantable pulse generator and two stimulation leads
The stimulator and leads are implanted in a minimally invasive, single-stage procedure involving two 3–5 cm skin incisions in the posterior lumbar-sacral region through the adipose tissue. The lead placement incision is midsagittal, approximately at the level of the L4 vertebral body, and the second incision is located at the insertion site for the IPG.
The leads are introduced percutaneously under fluoroscopic visualization using a needle, guide wire, and delivery sheath with dilator (Modified Seldinger approach) and distally anchored by deploying two opposing sets of flexible tines on either side of the L2/3 intertransversarii. Leads are placed bilaterally, with the electrodes positioned adjacent to the medial branch of the dorsal ramus as it crosses the transverse process at L3 (Figure 3). Correct position is verified both radiographically and via intraoperative electrical stimulus eliciting muscle twitch.
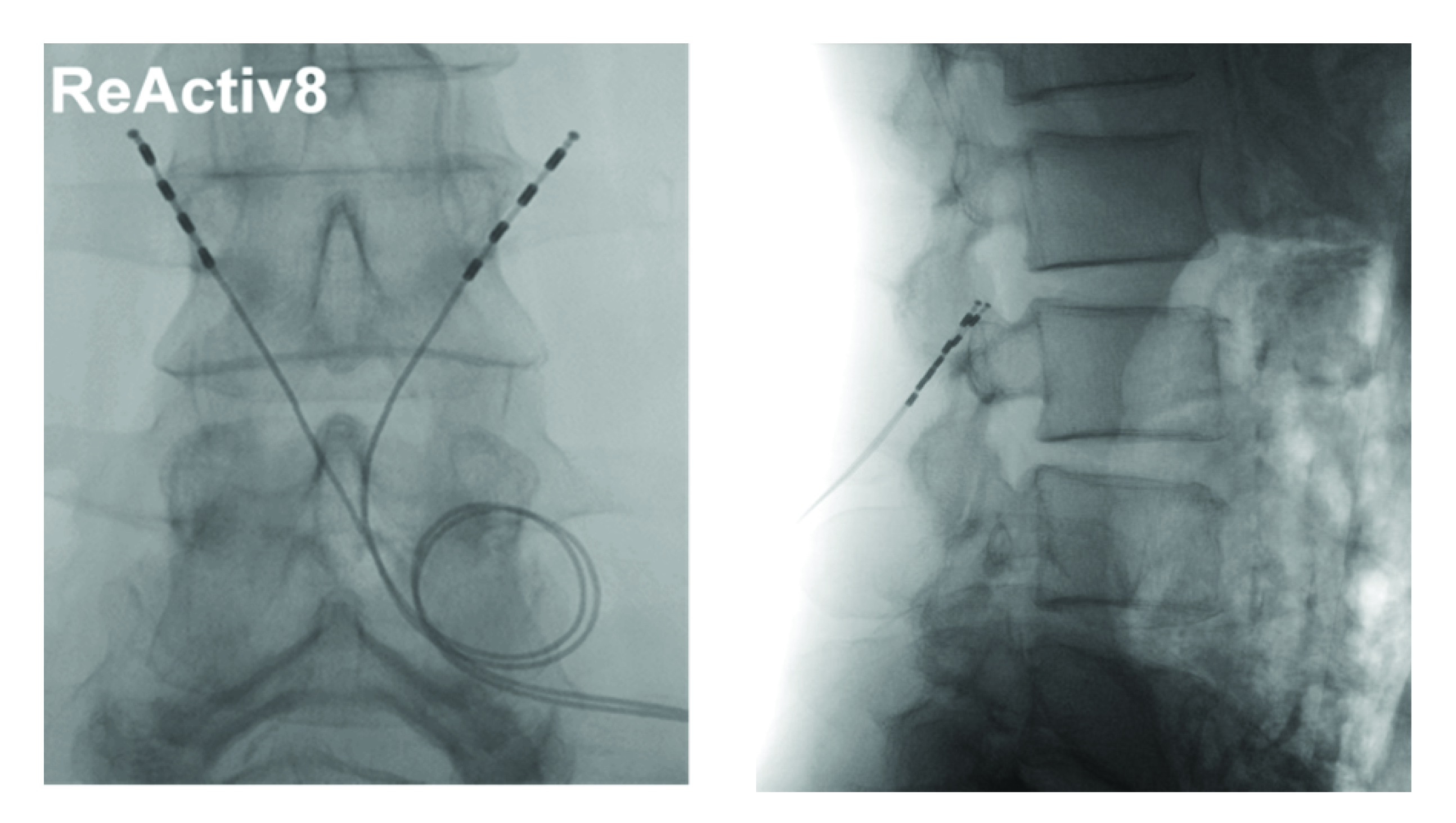 Figure 3. Anteroposterior and lateral view of implanted leads
Figure 3. Anteroposterior and lateral view of implanted leads
ReActiv8 leads are placed outside the spinal canal, adjacent to the peripheral nerve that innervates the multifidus muscle.
Leads are tunnelled subcutaneously between the lead implant incision and IPG pocket, and the terminals are inserted into the IPG header. The IPG is implanted in a subcutaneous pocket, either in the low back or high buttock region, no different from other implantable neurostimulation systems.
The patient initiates the stimulation sessions typically twice daily using the activator. During a session, the ReActiv8 system delivers 10-second trains of electrical stimulation twice per minute. The device does not deliver any stimulation between sessions. Detailed timing, stimulation amplitude, and frequency are programmed into the IPG when the device is placed and may be adjusted in the clinic during follow-up.
Relevance to Interventional Pain Practice
Restorative neurostimulation is an effective, durable, and safe treatment for patients with intractable, activity-limiting mechanical CLBP for whom available treatment options have provided insufficient relief or durability.
This novel treatment option is truly complementary because it fills an unmet clinical need of patients with refractory non-neuropathic CLBP. It is not an alternative for spinal cord stimulation that has long been the leading treatment option for radicular (neuropathic) CLBP, nor is spinal cord stimulation considered appropriate for non-neuropathic CLBP.10

Christopher Gilligan, MD, MBA, is chief of the division of Pain Medicine and vice chair for Strategy in the department of Anesthesiology, Perioperative, and Pain Medicine at Brigham and Women’s Hospital in
Boston, MA.
References
Russo M, Deckers K, Eldabe S, et al. Muscle control and non-specific chronic low back pain. Neuromodulation 2018;21(1):1–9. https://doi.org/10.1111/ner.12738.
Kim CW, Gottschalk LJ, Eng C, et al. The multifidus muscle is the strongest stabilizer of the lumbar spine. Spine J 2007;7:76S. https://doi.org/10.1016/j.spinee.2007.07.187.
Ward SR, Kim CW, Eng CM, et al. Architectural analysis and intraoperative measurements demonstrate the unique design of the multifidus muscle for lumbar spine stability. J Bone Joint Surg Am 2009;91(1):176–85. https://doi.org/10.2106/JBJS.G.01311.
Silfies SP, Mehta R, Smith SS, et al. Differences in feedforward trunk muscle activity in subgroups of patients with mechanical low back pain. Arch Phys Med Rehabil 2009;90(7):1159–69. https://doi.org/10.1016/j.apmr.2008.10.033.
Kim CW, Ward SR, Tomiya A, et al. Microarchitecture studies of the human multifidus muscle reveal its unique design as a major dynamic stabilizer of the lumbar spine. Poster presented at: 54th Annual Meeting of the Orthopaedic Research Society; March 2–5, 2008; San Francisco, CA. http://muscle.ucsd.edu/More_HTML/abstracts/ORS2008-Kim.pdf.
Carragee EJ, Cheng I, Freeman B, MJ. Minimum acceptable outcomes and expectations after fusion for degenerative disc disease. Spine J 2006;6(5):30S–1S. https://doi.org/10.1016/j.spinee.2006.06.394.
Rathmell JP. A 50-year-old man with chronic low back pain. JAMA 2008;299(17):2066–77. https://doi.org/10.1001/jama.299.13.jrr80002.
Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 2017;166(7):480–92. https://doi.org/10.7326/M16-2458.
Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2017;166(7):514–30. https://doi.org/10.7326/M16-2367.
Thomson S, Huygen F, Prangnell S, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. Eur J Pain 2020;24(6):1169–81. https://doi.org/10.1002/ejp.1562.
Hodges PW, Danneels L. Changes in structure and function of the back muscles in low back pain: different time points, observations, and mechanisms. J Orthop Sports Phys Ther 2019;49(6):464–76. https://doi.org/10.2519/jospt.2019.8827.
Massé-Alarie H, Flamand VH, Moffet H, et al. Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments. Exp Brain Res 2012;218(1):99–109. https://doi.org/10.1007/s00221-012-3008-9.
Hides JA, Jull GA, Richardson CA. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine 2001;26(11):E243–E248. http://www.ncbi.nlm.nih.gov/pubmed/11389408.
Deckers K, De Smedt K, Mitchell B, et al. New therapy for refractory chronic mechanical low back pain—restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodulation 2018;21(1):48–55. https://doi.org/10.1111/ner.12741.
Deckers K, De Smedt K, van Buyten J-P, et al. Chronic low back pain: restoration of dynamic stability. Neuromodulation 2015;18(6):478–86. https://doi.org10.1111/ner.12275.
Leave a commentOrder by
Newest on top Oldest on top