Buprenorphine Treatment Recommendations for Patients With Opioid Use Disorder
Cite as: Michaud K, Plunkett H, Kohan L Buprenorphine treatment recommendations for patients with opioid use disorder. ASRA News 2021;46. https://doi.org/10.52211/asra110121.064
Opioid use disorder (OUD) is a health crisis in the United States,1 but medication treatment of OUD (MOUD) along with psychosocial counseling and behavioral treatments,2 has been shown to improve outcomes, increase retention, and decreased morbidity and mortality.3 Three medications have current U.S. Food and Drug Administration (FDA) approval for MOUD: methadone, buprenorphine, and naltrexone, all of which are similarly efficacious. This article discusses new guidelines for buprenorphine MOUD therapy.
Buprenorphine for MOUD
Hospitalizations and death rates related to opioid overdose are increasing.4 Medical management, in addition to other treatment modalities, saves lives in patients with OUD.5 However, inadequate patient and physician education, federal and other restrictive regulations, stigma regarding therapy, inadequate insurance coverage, and lack of coordination among care specialists have been reported as barriers to MOUD treatment.5–7
Pharmacologic Principles of Buprenorphine
Buprenorphine is a partial mu-opioid agonist with high-affinity binding but a ceiling effect that reduces euphoria and respiratory depression. It is also a partial kappa-opioid agonist or functional antagonist with possible antidepressant effects. Naloxone may be added in some preparations to decrease injection misuse. Buprenorphine may be administered sublingually, transmucosally, intramuscularly, or transdermally8 (Table 1).
Table 1. Opioid Use Disorder Medications24,38–42
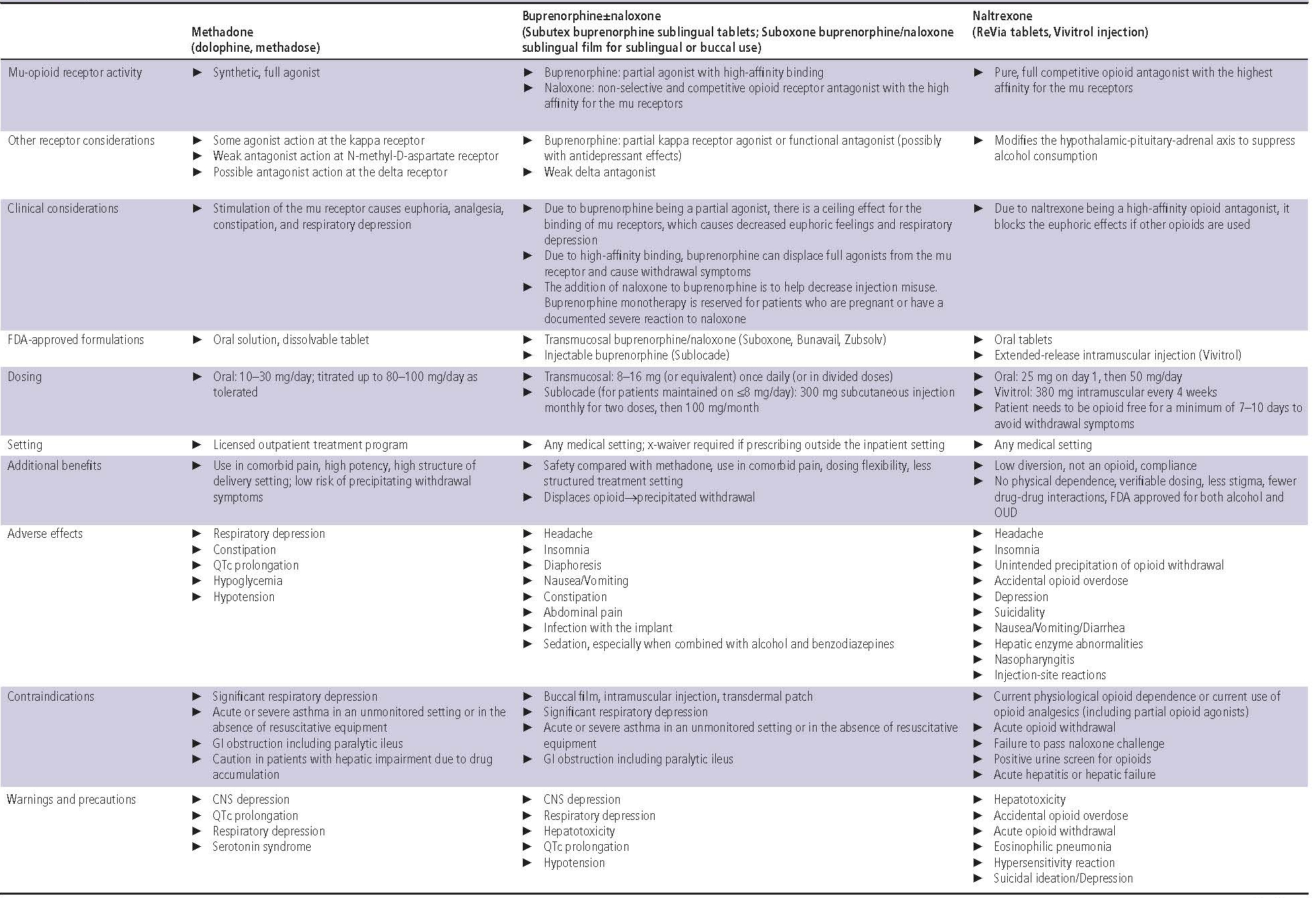

Bioavailability of buccal film administration is 46%–65%, intramuscular injection 70%, sublingual tablets 29%, and transdermal patch 15%. Onset of action is greater than 15 minutes. Buprenorphine is metabolized by CYP3A4 to norbuprenorphine, an active metabolite, which undergoes glucuronidation. It is excreted in the feces and urine. The half-life is 28 + 11 hours for buccal films, 37 hours for sublingual tablets, and 26 hours for transdermal patches8 (Table 1).
Dosing varies depending on the application. Formulations with FDA approval for OUD use milligram dosing, whereas formulations with approval for chronic pain use microgram dosing. When used for OUD, buprenorphine is available parenterally as an extended-release subcutaneous injection and sublingually as a tablet or film8 (Table 1).
Common side effects of buprenorphine treatment include headache, insomnia, diaphoresis, nausea, vomiting, constipation, abdominal pain, infection with implant, and sedation, especially when combined with alcohol or benzodiazepines. Contraindications include existent significant respiratory depression, acute or severe asthma in an unmonitored setting, and gastrointestinal obstruction, including paralytic ileus8 (Table 1).
Misconceptions About Buprenorphine
Some providers have misconceptions regarding the use of buprenorphine as an analgesic agent. It is sometimes mistakenly regarded as a weak analgesic9—or that it will decrease the effectiveness of a full mu-opioid agonist, also resulting in potential inadequate analgesia. In actuality, buprenorphine was first developed as an analgesic.10 When it is administered in maintenance doses for OUD, mu-opioid binding sites remain available. When treating acute pain, however, it is essential to maximize nonopioid and nonpharmacologic agents because opioids alone may be insufficient for high levels of postsurgical pain.8
Buprenorphine carries inherently less potential for misuse and more protection from overdose than methadone.11 It is also associated with fewer withdrawal symptoms and cravings, less sedation, lower risk of toxicity at higher doses, and protection in overdose situations when compared with methadone.12,13
Perioperative Management of Chronic Buprenorphine Therapy for MOUD
An increasing number of patients are presenting for surgery while on buprenorphine therapy for OUD, presenting unique challenges and opportunities for anesthesiologists and acute pain services.14 During perioperative planning, physicians may have concerns with treatment of postoperative pain in patients on buprenorphine therapy given that a full mu-opioid agonist is unable to displace buprenorphine because of its high affinity for the mu-opioid receptor, ability to displace full mu-opioid agonists, and its long half-life.15
In 2004, the U.S. Center for Substance Abuse Treatment recommended the general discontinuation of buprenorphine while patients are taking full mu-opioid agonists.16 More recent evidence suggested that even though buprenorphine has high affinity at the mu-opioid receptor, some receptors remain unoccupied and are available to bind full mu-opioid agonists; thus, buprenorphine in combination with full mu-opioid agonists may effectively treat acute and perioperative pain.17–21 Because of those findings, perioperative management of patients on buprenorphine therapy is evolving away from traditional teaching of holding the agent to “open up receptors.” Newer consensus recommends continuing buprenorphine, without the addition of naloxone, throughout the perioperative period,18 further supported by evidence suggesting that the discontinuation of buprenorphine may be harmful and, in some cases, even fatal.22
The current consensus recommends8:
- Preoperative planning for patients on chronic buprenorphine therapy: Neither discontinue nor taper high-dose buprenorphine prior to surgery (Grade B, moderate level of certainty).
- Postoperative pain management in patients on chronic buprenorphine therapy: Use a multimodal analgesia, including adjunctive medications and regional anesthesia (Grade B, moderate level of certainty) (Figure 1).
- Discharge planning for patients on chronic buprenorphine therapy: Studies suggest the importance of providing an opioid taper-off plan with appropriate surgical prescribing and postoperative follow-up, which can minimize withdrawal, improve successful discontinuation, and decrease overall postsurgical opioid use. If doses were increased in the perioperative period to aid with acute pain, return to the preoperative maintenance dose of buprenorphine (Grade A, moderate level of certainty). Based on length of expected recovery and the patient’s preoperative illicit opioid use, taper entirely off full mu-opioid agonists prescribed for acute postoperative pain (Grade A, moderate level of certainty). Special consideration is given and caution advised for patients who have active or recent illicit opioid abuse. In all circumstances, engage the patient in an ongoing collaboration with an outpatient buprenorphine prescriber (Grade A, moderate level of certainty).

Figure 1. Recommendations for Postoperative Management With Buprenorphine and Other Options
Principles of Inpatient Buprenorphine Treatment for MOUD
Substance abuse, including use of tobacco, alcohol, and illicit drugs, is common among hospitalized patients.23 Death rates among patients with OUD are highest in the first month following a hospital discharge.24 Patients with opioid dependence are at increased risk for general health-related events and therefore seek frequent emergency department care.25 Those encounters offer physicians the opportunity to intervene and positively affect patients26 by initiating buprenorphine therapy either as inpatient care or upon discharge to treat OUD, thereby potentially saving lives.27 Patients who are initiated on buprenorphine therapy during a hospitalization have greater long-term MOUD use, decreased use of inpatient OUD treatment services, and reduced self-reported illicit opioid use.28,29
Establishing inpatient collaboration and alliances with community programs and outpatient primary care practices can assist in streamlining patients’ postdischarge care.30 Unfortunately, efficient inpatient OUD services are limited. One strategy to increase efficient MOUD is initiating buprenorphine without requiring prior withdrawal symptoms, which has shown to be safe for perioperative patients with suspected OUD (Figure 2).31
 Figure 2. Perioperative Buprenorphine Therapy
Figure 2. Perioperative Buprenorphine Therapy
Even a short course of MOUD for patients with OUD improves survival. Buprenorphine-assisted detoxification has been shown to decrease mortality, even when outpatient follow-up was not achieved.32 Because MOUD reduces risk of overdose, physicians are encouraged to begin patients on treatment as standard practice.33,34 Some states mandate the treatment of OUD in emergency departments (EDs),35 because many patients who began buprenorphine therapy for OUD in the ED remained engaged in long-term therapy after discharge.36
Buprenorphine vs Methadone for Outpatient MOUD Treatment
Buprenorphine may be effectively prescribed and managed in an office-based setting,37 whereas methadone must be dispensed from a specialized methadone clinic.12 When prescribing buprenorphine, an x-waiver or a notice of intent to obtain an x-waiver without training is required.2,12 Physicians can prescribe buprenorphine in the inpatient setting without special licensure, but they must obtain a regulatory license to prescribe methadone. Methadone clinics also require specialized operations licensure, and the Narcotic Addict Treatment Act of 1974 restricts all access to methadone.12 Buprenorphine may be obtained from a traditional outpatient pharmacy and is either dosed once daily or multiple times daily in divided doses, per patient preference. Conversely, methadone must be obtained from a methadone clinic daily.8
The authors would like to thank the members of the Ad-Hoc OUD Working Group (listed below) for their contributions, as well as ASRA staff members Angie Stengel, Anne Snively, and Athena Ermidis.
- Eugene Viscusi, MD, ASRA Immediate Past President
- Lynn Kohan, MD, Working Group Chair
- Olabisi Lane, MD, and Sudheer Potru, DO, ASRA
- Trent Emerick, MD, American Academy of Pain Medicine and American Society of Addiction Medicine
- Michael Sprintz, DO, American Society of Addiction Medicine
- Anuj Aryal, MD, and Antje Barreveld, MD, American Society of Anesthesiologists
- Sophia Chhay, PharmD, and Anna Dopp, PharmD, American Society of Health-System Pharmacists

Kristina Michaud, DO, is an anesthesiology resident at the University of Virginia in Charlottesville.
Harrison Plunkett, MD, is an anesthesiologist chief resident at the University of Virginia in Charlottesville.
Lynn Kohan, MD, is an associate professor of anesthesiology and pain medicine at the University of Virginia in Charlottesville.
References
- Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths— United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65:1445–52.
- Bart G. Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis 2012;31:207–25. https://doi.org/10.1080/10550887.2012.694598
- Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017;357:j1550. https://doi.org/10.1136/bmj.j1550
- Englander H, Weimer M, Solotaroff R, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med 2017;12:339–42.
- National Academies of Sciences, Engineering, and Medicine. Medications for opioid use disorder save lives. Washington, DC: National Academies Press; 2019.
- Mendoza S, Rivera-Cabrero AS, Hansen H. Shifting blame: buprenorphine prescribers, addiction treatment, and prescription monitoring in middle-class America. Transcult Psychiatry 2016;53:465–87. https://doi.org/10.1177/1363461516660884
- Huskamp HA, Riedel LE, Barry CL, et al. Coverage of medications that treat opioid use disorder and opioids for pain management in marketplace plans, 2017. Med Care 2018;56(6):505–9. https://doi.org/10.1097/MLR.0000000000000918
- Kohan L, Potru S, Barreveld A, et al. Buprenorphine management in the perioperative period: educational review and recommendations from a multisociety expert panel. Reg Anesth Pain Med 2021;46(10):840–59. https://doi.org/10.1136/rapm-2021-103007
- Virk MS, Arttamangkul S, Birdsong WT, et al. Buprenorphine is a weak partial agonist that inhibits opioid receptor desensitization. J Neurosci 2009;29:7341–48. https://doi.org/10.1523/jneurosci.3723-08.2009
- Campbell ND, Lovell AM. The history of the development of buprenorphine as an addiction therapeutic. Ann NY Acad Sci 2012;1248:124–39. https://doi.org/10.1111/j.1749-6632.2011.06352.x
- Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract 2012;3:45–50. https://doi.org/10.4103/0976-3147.91934
- Welsh C, Valadez-Meltzer A. Buprenorphine: a (relatively) new treatment for opioid dependence. Psychiatry (Edgmont) 2005;2:29–39. https://pubmed.ncbi.nlm.nih.gov/21124750
- Shulman M, Wai JM, Nunes EV. Buprenorphine treatment for opioid use disorder: an overview. CNS Drugs 2019;33:567–80. https://doi.org/10.1007/s40263-019-00637-z
- Coluzzi F, Bifulco F, Cuomo A, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag 2017;13:1163–73. https://doi.org/10.2147/tcrm.s141332
- AHFS DI Essentials. American Society of Health-System Pharmacists. Bethesda, MD; 2021. https://www.ahfsdruginformation.com. Accessed October 18, 2021.
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment improvement protocol (TIP) series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health; 2004.
- Macintyre PE, Russell RA, Usher KA, et al. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care 2013;41:222–30. https://doi.org/10.1177/0310057x1304100212
- Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: a case series. Am J Ther 2010;17:523–28. https://doi.org/10.1097/mjt.0b013e3181be0804
- Silva MJ, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliat Care Pharmacother 2016;30:289–93. https://doi.org/10.1080/15360288.2016.1231734
- Vilkins AL, Bagley SM, Hahn KA, et al. Comparison of post-Cesarean section opioid analgesic requirements in women with opioid use disorder treated with methadone or buprenorphine. J Addict Med 2017;11:397–401. https://doi.org/10.1097/adm.0000000000000339
- Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician 2009;12:679–84. https://pubmed.ncbi.nlm.nih.gov/19461836
- Warner NS, Warner MA, Cunningham JL, et al. A practical approach for the management of the mixed opioid agonist-antagonist buprenorphine during acute pain and surgery. Mayo Clin Proc 2020;95:1253–67. https://doi.org/10.1016/j.mayocp.2019.10.007
- Katz A, Goldberg D, Smith J, et al. Tobacco, alcohol, and drug use and willingness to change. J Hosp Med 2008;3:369–75. https://doi.org/10.1002/jhm.358
- White SR, Bird SM, Merrall EL, et al. Drugs-related death soon after hospital-discharge among drug treatment clients in Scotland: record linkage, validation, and investigation of risk-factors. PLOS One 2015;10:e0141073. https://doi.org/10.1371/journal.pone.0141073
- Hansen LE, Stone GL, Matson CA, et al. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty 2016;31:1698–701. https://doi.org/10.1016/j.arth.2016.01.032
- Patel N, Schwenk ES, Ferd P, et al. An anesthesiologist-led inpatient buprenorphine initiative. Pain Pract 2021;21(6):692–97. https://doi.org/10.1111/papr.12996
- Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018;169(3):137–45. https://doi.org/0.7326/M17-3107
- D’Onfrio G, O-Connor P, Pantalon M, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized control clinical trial. JAMA 2015;313:1636–44. https://doi.org/10.1001/jama.2015.3474
- Liebschutz J, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid dependent patients: a randomized clinical trial. JAMA Intern Med 2014;174:1369–76. https://doi.org/10.1001/jamainternmed.2014.2556
- Madras BK, Ahmad NJ, Wen J, et al. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. Washington, DC: National Academies Press; 2020. https://doi.org/10.31478/202004b
- Hämmig R. Einleitung einer substitutionsbehandlung mit buprenorphin unter vorübergehender überlappung mit heroinkonsum: ein neuer ansatz (“Berner methode”). Suchttherapie 2010;11:129–32. https://doi.org/10.1055/s-0030-1261914
- Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction 2015;110(6):996–1005. https://doi.org/10.1111/add.12863
- Cornish R, Macleod J, Strang J, et al. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ 2010;341:c5475. https://doi.org/10.1136/bmj.c5475
- Houry DE, Haegerich TM, Vivolo-Kantor A. Opportunities for prevention and intervention of opioid overdose in the emergency department. Ann Emerg Med 2018;71:688–90.
- American
College of Emergency Physicians. State legislation requiring emergency
medication assisted therapy for opioid use disorder. Accessed April 5, 2021. https://www.acep.org/how-we-serve/sections/toxicology/news/september-2018/state-legislation-requiring-emergency-medication-assisted-therapy-for-opioid-use-disorder
- Kaucher KA, Caruso EH, Sungar G, et al. Evaluation of an emergency department buprenorphine induction and medication-assisted treatment referral program. Am J Emerg Med 2020;38:300–4. https://doi.org/10.1016/j.ajem.2019.158373
- Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med 2008;148:662–70. https://doi.org/10.7326/0003-4819-148-9-200805060-00006
- Coffa
D, Harter K, Smith B, et al. Acute pain and perioperative management in opioid
use disorder: pain control in patients on buprenorphine, methadone,
or naltrexone. SHOUT Guidelines. Published
April 19, 2008. Accessed September 20, 2020. https://drive.google.com/drive/folders/1TYvygcY2FcQjvdgEjfvo4JtEvkkOxBe4
- Pierce M, Bird SM, Hickman M, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction (Abingdon, England) 2016;111(2):298–308. https://doi.org/10.1111/add.13193
- Birnbaum HG, White AG, Schiller M, et al. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med (Malden, Mass) 2011;12:657–67. https://doi.org/10.1111/j.1526-4637.2011.01075.x
- White AG, Birnbaum HG, Mareva MN, et al. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm 2005;11:469–79. https://doi.org/10.18553/jmcp.2005.11.6.469
- Wang S. Historical review: opiate addiction and opioid receptors. Cell Transplant 2019;28(3):233–38. https://doi.org/10.1177/0963689718811060


Leave a commentOrder by
Newest on top Oldest on top