The Future of Pain Medicine, Is It Surgical?
Cite as: Xie S, Mukhdomi T, Eshraghi Y. The future of pain medicine, is it surgical?. ASRA Pain Medicine News 2022;47. https://doi.org/10.52211/asra050122.022
Over the past few years, interventional pain medicine has had a renaissance in new technologies, beyond intrathecal pumps and traditional spinal cord stimulators (SCS). It has entered a realm of more minimally invasive surgical procedures, such as basivertebral nerve ablation, dorsal root ganglion stimulator (DRGS), interspinous fixation device, interspinous spacer, minimally invasive lumbar decompression (MILD), minimally invasive sacroiliac joint (SIJ) fusion, peripheral nerve stimulator, and restorative implantable neurostimulator.
These newer modalities advance the treatment algorithm for pain with the potential for more definitive options, especially in cases where conservative treatments have failed. Unfortunately, not all training programs have grown with the advances, as such advances may be premature to adopt and involve politics with encroaching on surgical territory. Perhaps the conservative nature of academic training leads to refraining from early adoption until a modality has been well researched and widely accepted.
Benefits and Risks
Minimally invasive interventions have grown in popularity due to their advantages over open surgeries. When comparing MILD and interspinous spacers to open surgical decompression, patients benefited from reductions in procedure and recovery time, anesthetic need and risk, hospitalization, soft tissue injury and blood loss, and complication rates1 (Figure 1). These procedures can be performed in an outpatient setting under minimal anesthesia, which is beneficial to patients who are poor surgical candidates but have symptomatic lumbar spinal stenosis (LSS) refractory to conservative treatments. Recent studies on minimally invasive sacroiliac joint (SIJ) fusion vs open approach showed “lower rates of revision surgery at 30- and 180-day readmission and lower postoperative rates of lumbar pathology at all timepoints. In addition, the same patients had lower opioid usage rates at 2 years postoperatively compared with those receiving conservative care.”2
These interventions are not without risks. Adverse events of interspinous spacers include spinous or transverse process fracture, device migration and malfunction, need for reoperation, infection, cerebrospinal fluid leak, pain, and worsening of symptoms3 (Figure 1). Reported procedure-related adverse events of intraosseous basivertebral nerve ablation include nerve root injury, lumbar radiculopathy, retroperitoneal hemorrhage, and transient motor or sensory deficits that occurred and resolved within the perioperative period.4 Complications of DRGS include dural puncture, neurologic injury, infection, lead damage, and lead migration; although there is ongoing development in anchoring techniques to minimize lead migration5 (Figure 1).
Despite the risks due to their invasive nature (compared to conservative treatments) and the need for more clinical trials to evaluate their safety and efficacy, these interventions can make a significant difference in patients’ lives with the potential to reduce opioid requirements, improve quality of life and functional capacity, and reduce the psychological effects of pain.
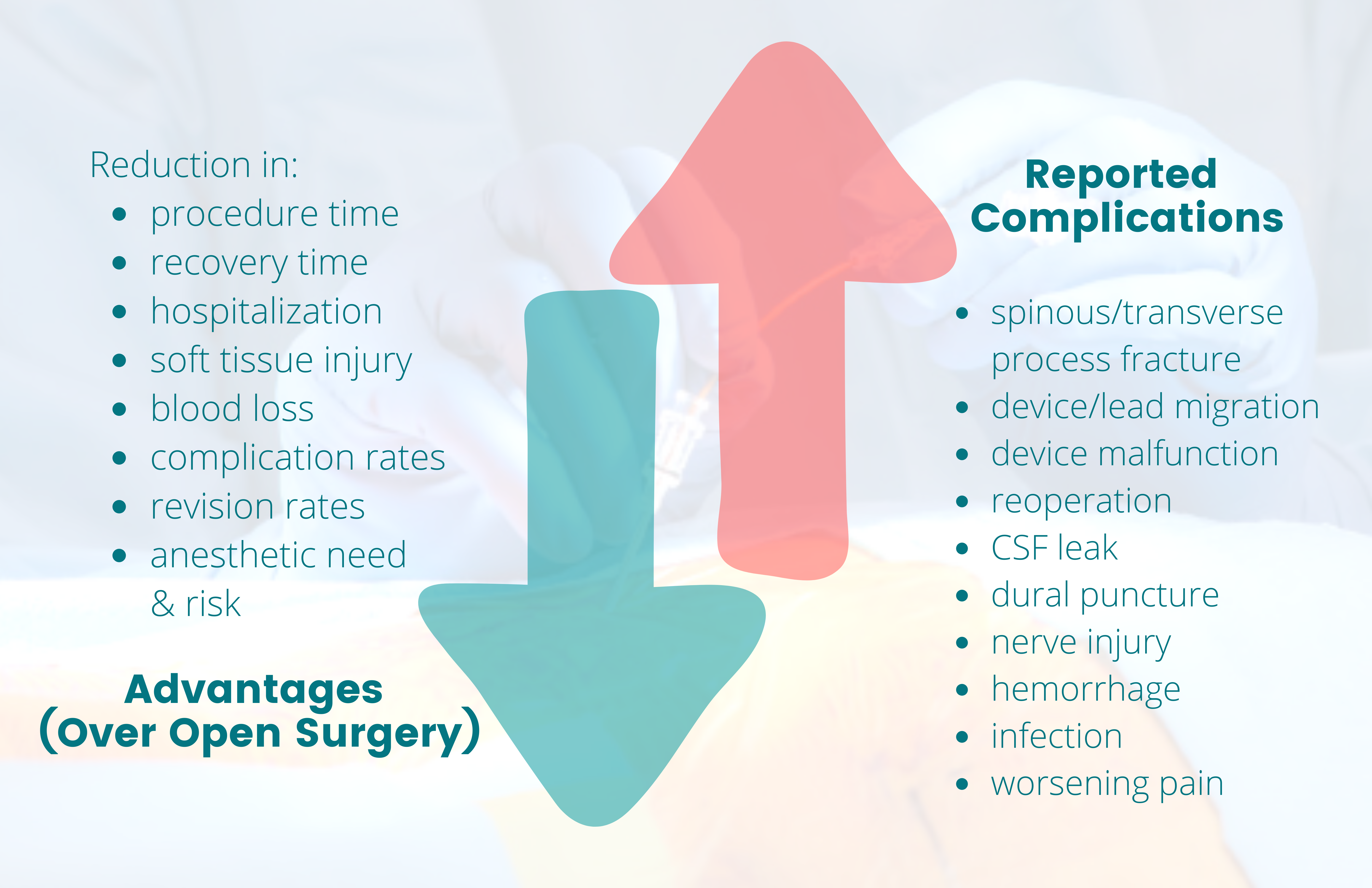
Figure 1. Benefits vs risk of minimally invasive interventions
The green down arrow corresponds with the list of various reductions that highlight the advantages (benefits) of minimally invasive interventions over open surgery. The red up arrow corresponds with the list of reported complications (risks) of these interventions that would not have otherwise occurred with conservative management only.
Cost Effectiveness
These minimally invasive interventions have higher initial costs than conservative treatments (but lower costs than open surgery); however, they have the potential to be cost-effective over time if the results are successful.
In a 2-year cost-effectiveness study of epidural steroid injections (ESI) vs MILD vs laminectomy in treating lumbar spinal stenosis (LSS), MILD had a higher initial cost than ESI but less than laminectomy. MILD appeared to be the most cost-effective at $43,760 per quality-adjusted life years (QALY), while laminectomy was least cost-effective at $125,985/QALY. ESI had an incremental cost-effectiveness ratio (ICER) of $37,758 per QALY and would only be more cost effective than MILD if the number of ESIs in a 2-year period was 6 or fewer.6
In another 2-year cost-effectiveness study of conservative care (CC) vs laminectomy vs the interspinous spacer in treating LSS, CC had the lowest cost ($10,540) but the lowest QALY increase (0.06), while the interspinous spacer had an ICER of $16,302 per QALY gained, and laminectomy had an ICER of $15,231 per QALY gained.7
In a cost-effectiveness study using a 10-year Markov cohort model to analyze DRGS vs SCS vs comprehensive medical management (CMM) in treating complex regional pain syndrome (CRPS-I and II), DRGS had the highest cost vs SCS and CMM ($153,992 vs $128,269 and $106,173, respectively), but both DRGS and SCS were cost-effective ($34,695 per QALY and $22,084 per QALY respectively) compared to CMM.8
There are limitations to the above studies but given the potential cost-saving measures and benefits of such interventions, perhaps reviewing treatment algorithms to include earlier intervention with advanced procedures can lead to decreased healthcare costs, earlier improvement in functional status and pain, and quicker return to a better quality of life. The novel approach to introduce advanced procedures early on can be especially beneficial to those interventions with trial periods where patients can experience the device temporarily before proceeding with the permanent implant, thus alleviating apprehension early in the treatment paradigm.
Discussion
These new interventions and devices can have promising results in early trials, but because they are new, it may be difficult to fully analyze their safety profile and outcome. Long-term and well-designed follow-up trials will be helpful to evaluate the efficacy of new interventions.
Early studies of X-Stop, an interspinous spacer approved by the US Food and Drug Administration in 2005, showed significant improvement in symptoms and quality of life over conservative treatment in short-term results; however, the two primary authors reportedly had a conflict of interest and inflated the positive outcomes of their studies.9 A small series study on X-Stop with a 4-year follow up showed a failure rate with the need for additional spinal surgery of 85% (11 of 13 patients),10 and a study in Norway showed that X-Stop had higher rates of reoperation for persistent symptoms compared to minimally invasive surgery decompression techniques.11 Eventually X-Stop was taken off the market due to its adverse events profile.3
Similar issues were found with MILD; however, these concerns were rectified with the MiDAS Study, thus reinstating the procedure as a Medicare-covered intervention.12 When compared to patients treated only with ESIs, the 2-year results of this study showed that patients treated with MILD had clinically and statistically significant pain reduction and functional improvement with no evidence of spinal instability or serious device-/procedure-related adverse events, while having lower reoperation and spinal fracture rates than other lumbar spine interventions such as surgical decompression and spinal fusion.12 An argument can be made that the interventional pain specialist community is demonstrating that we as clinicians are learning from prior pitfalls and justifying our efforts to advance therapeutic interventions with reliable studies and outcomes to support new advances.
Provider Training for Advanced Interventions
Procedural training can vary widely among academic training programs. A survey study by Pak et al. examined the presence of SCS training for pain fellowships and concluded that although training has increased, experiences are highly variable and “most rely on industry-sponsored programs to supplement training deficiencies.”13 In 2011, Wright et al. surveyed program directors of all accredited U.S. pain medicine fellowships. They reported that 78% of respondents “felt pertinent surgical training should be mandatory before credentialing, but less than 20% reported having been required to even have proctored experience before credentialing.”14 A decade later, training programs are adapting to the growth in practice. Device companies are starting education programs and approval processes. Certificates for physicians are being provided for advanced procedures to ensure patient safety and demonstrate operator competency, termed ‘credentialing’.15
Interventional pain medicine training should include exposure to advanced surgical pain procedures. This allows pain fellows to develop these skill sets. As pain fellows practice independently as physicians, they can decide if they will incorporate this into their personal practice.
Conclusion
Minimally invasive procedures are becoming an integral part of pain medicine. It is important that we evaluate and adopt these new technologies while reviewing the evidence and following the successes as well as complications based on real-world experiences.16 Overall, the importance of vetting innovative procedures prior to widespread adaptation is paramount to best serve our current patients and advancement of our field as interventionalists.

“Sisi” Siyun Xie, MD, is a PGY3 anesthesiology resident at the University of Texas Medical Branch in Galveston, TX.
Taif Mukhdomi, MD, is an interventional pain medicine fellow at the New York Presbyterian/Weill-Cornell Medical Center, Memorial Sloan-Kettering Cancer Center, and Hospital for Special Surgery, Tri-Institutional Pain Fellowship in New York, NY.
Yashar Eshraghi, MD, is the medical director of Pain Research, assistant program director of the Pain Medicine Fellowship Training Program, and associate professor in the department of Anesthesiology and Critical Care Medicine at Ochsner Health System at the University of Queensland Ochsner Medical School. He is also a clinical assistant professor at Louisiana State University School of Medicine in New Orleans.
References
- Hartman J, Granville M, Jacobson RE. The use of Vertiflex® interspinous spacer device in patients with lumbar spinal stenosis and concurrent medical comorbidities. Cureus 2019;11(8):e5374. https://doi.org/10.7759/cureus.5374
- Ballatori AM, Shahrestani S, Chen XT, et al. Propensity-matched analysis of 1062 patients following minimally invasive versus open sacroiliac joint fusion. Clin Spine Surg 2021;34(8):E477-E482. https://doi.org/10.1097/BSD.0000000000001244
- Aggarwal N, Chow R. Real world adverse events of interspinous spacers using manufacturer and user facility device experience data. Anesth Pain Med (Seoul) 2021;16(2):177-83. https://doi.org/10.17085/apm.20093
- Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2018;27(5):1146-56. https://doi.org/10.1007/s00586-018-5496-1
- Sivanesan E, Bicket MC, Cohen SP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg Anesth Pain Med 2019;44(1):100-6. https://doi.org/10.1136/rapm-2018-000007
- Udeh BL, Costandi S, Dalton JE, et al. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients. Pain Pract 2015;15(2):107-16. https://doi.org/10.1111/papr.12160
- Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: Conservative care, laminectomy, and the Superion interspinous spacer. Int J Spine Surg 2015;9:28. https://doi.org/10.14444/2028
- Mekhail N, Deer TR, Poree L, et al. Cost-effectiveness of dorsal root ganglion stimulation or spinal cord stimulation for complex regional pain syndrome. Neuromodulation 2021;24(4):708-18. https://doi.org/10.1111/ner.13134
- Gazzeri R, Galarza M, Alfieri A. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: past, present, and future. Biomed Res Int 2014;975052. https://doi.org/10.1155/2014/975052
- Bowers C, Amini A, Dailey AT, Schmidt MH. Dynamic interspinous process stabilization: review of complications associated with the X-Stop device. Neurosurg Focus 2010;28(6):E8. https://doi.org/10.3171/2010.3.FOCUS1047
- Huddleston P. X-stop resulted in a higher reoperation rate than minimally invasive decompression, but both decreased symptoms of neurogenic intermittent claudication in patients with lumbar spinal stenosis. J Bone Joint Surg Am 2018;97(22):1889. https://doi.org/10.2106/JBJS.9722.ebo101
- Staats PS, Chafin TB, Golovac S, et al. MiDAS ENCORE investigators. long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med 2018;43(7):789-94. https://doi.org/10.1097/AAP.0000000000000868
- Pak DJ, Gruber J, Deer T, et al. Spinal cord stimulator education during pain fellowship: unmet training needs and factors that impact future practice. Reg Anesth Pain Med 2019;44(3):407-14. https://doi.org/10.1136/rapm-2018-100065
- Wright TB, Enu I, Stansbury LG, et al. Interventional pain physicians' experiences of and attitudes toward surgical privileging. Reg Anesth Pain Med 2011;36(5):457-60. https://doi.org/10.1097/AAP.0b013e318219e23e
- Naidu RK, Chaturvedi R, Engle AM, et al. Interventional Spine and Pain Procedure Credentialing: Guidelines from the American Society of Pain & Neuroscience. J Pain Res. 2021 Sep 8;14:2777-2791. doi: 10.2147/JPR.S309705.
- Goodman SB, Mihalko WM, Anderson PA, et al. Introduction of new technologies in orthopaedic surgery. JBJS Rev 2016;4(5):e5. https://doi.org/10.2106/JBJS.RVW.O.00067


Leave a commentOrder by
Newest on top Oldest on top