POCUS Spotlight: Subcostal Cardiac Ultrasound
Introduction
Recent years have seen a dramatic increase in the use of echocardiography in critical care and anesthesia, with focused cardiac ultrasound now a core competency in several countries.1-4 In mechanically ventilated and critically ill patients, the image quality acquired in the subcostal (SC) view tends to be better compared to those obtained through transthoracic echocardiographic windows.5–11 Traditional use of the SC window is largely limited to the SC four-chamber and longitudinal inferior vena cava (IVC) views. However, the SC window offers several alternative views that allow for an assessment of the heart across multiple planes.4,10,12-14
In mechanically ventilated and critically ill patients, the image quality acquired in the subcostal view tends to be better compared to those obtained through transthoracic echocardiographic windows.
When to Use the Subcostal Window
The SC window is recommended as a part of focused and comprehensive echocardiography in all patients.1,7,8 It may be particularly useful for assessment of the following.
The Pericardium
The SC window provides a global view of the pericardial sac, in more than one plane, and is easily accessible. Its ability to clearly visualize the right ventricular free wall allows for the detection of anterior pericardial effusions that are often not well visualized in other windows and signs of tamponade, such as chamber collapse (Figure 1).15
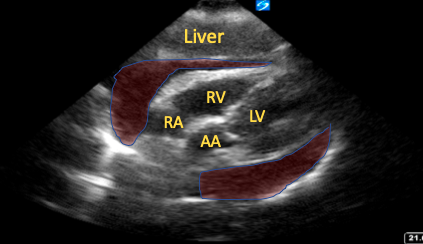
Figure 1. Subcostal four-chamber view with a large pericardial effusion (shaded red areas) and right atrial collapse visible.
RA=right atrium, RV=right ventricle, LV=left ventricle, AA=ascending aorta
Pericardium
The SC window can be used to guide pericardiocentesis and confirm response following treatment.16-18 The SC four-chamber view is obtained, the target pericardial fluid identified, and its depth from the skin estimated. The needle is inserted under ultrasound guidance in the subxiphoid region at 45o, directed toward the patient’s left scapula. Once the pericardial space is under direct vision, fluid is aspirated, and the needle position confirmed by the injection of agitated saline as contrast. A catheter can then be inserted.16-18
The Atria
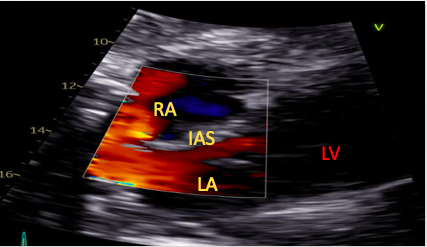
The standard SC four-chamber view provides clear visualization of both atria allowing for assessment of numerous pathologies including myxomas, masses, and atrial thrombi.19,20 The interatrial septum can also be visualized in this view and runs almost perpendicular to the ultrasound beam, allowing for a thorough assessment of septal pathology using both color flow Doppler interrogation and contrast echocardiography (Figure 2).21
Figure 2. Subcostal view with color assessing the interatrial septum for the presence of abnormal flow.
RA=right atrium, LA=left atrium, LV=left ventricle
The Right Ventricle
The SC view is the optimal window for assessment of the right ventricle (RV) free wall and RV thickness. RV wall thickness is measured at end-diastole, with hypertrophy commonly defined as a thickness of >5 mm. The thickness of the RV wall may help differentiate acute from chronic pathology.
The SC window also allows a functional assessment of RV function, which is particularly important if other views are not available or are of poor quality. Visual estimation of function is possible, as is assessment of RV effects on the interventricular septum, but it should be acknowledged that the RV is foreshortened in this view, limiting assessments of size.7,8,22 Quantitative assessment of longitudinal function is also possible using the SC echocardiographic assessment of tricuspid annular kick (SEATAK).15,22,23 To perform SEATAK, the SC short axis view is obtained, M-mode is placed over the tricuspid annulus, and its excursion (kick) measured (Figure 3). This is analogous to tricuspid annular plane systolic excursion (TAPSE) in the apical four-chamber window. There will likely be an element of misalignment with the M-mode cursor and movement of the tricuspid annulus. Thus, SEATAK distances are lower than the equivalent TAPSE, and, as a result, SEATAK is not well validated. 23
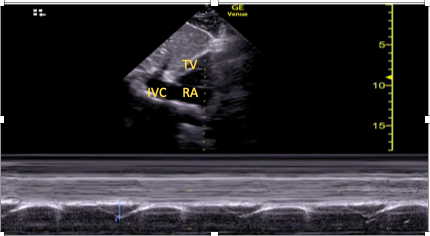
Figure 3. Example of subcostal echocariographic assessment of tricuspid annual kick being performed using the subcostal short axis window.
TV=tricuspid valve, RA=right atrium, IVC=inferior vena cava
The Left Ventricle
While the LV is most commonly assessed in the parasternal and apical windows, the SC view offers a useful alternative when these views are not available. A rough estimate of LV size and global function can be ascertained from the SC four-chamber view, and the addition of the SC short-axis view allows radial contraction to be assessed and regional wall motion abnormalities identified.
The Valves
The SC four-chamber view allows visualization of the mitral and tricuspid valves (although the near perpendicular alignment means Doppler assessment is unreliable). The SC five-chamber view can be used to visualize the LV outflow tract, aortic valve, and aortic root. The SC short-axis view can then be found, the tricuspid and pulmonary valves visualized (Doppler assessment may be possible here due to better alignment), and the aortic valve assessed in transverse section. Progression to SC short-axis mitral valve view allows assessment of the mitral valve leaflets.
The Inferior Vena Cava
The IVC can be clearly visualized from the SC window both in longitudinal and transverse sections (Figure 4). The IVC diameter is measured at approximately 1-2 cm proximal to the right atrial junction, with a diameter >2 cm suggestive of IVC distension and potentially raised right atrial pressures.24 Dynamic assessment of the IVC can be performed by assessing the change in its diameter throughout the respiratory cycle.24 In mechanically ventilated patients, the IVC “distensibility index” with respiration can be used, with a dIVC ≥18% associated with 90% specificity and sensitivity for fluid responsiveness.15, 24
The Aorta
The abdominal aorta can be visualized in transverse and longitudinal axes from the SC window. This can aid with the diagnosis of pathologies such as aortic aneurysms or dissections (visualized as a flap within the vessel).25,26
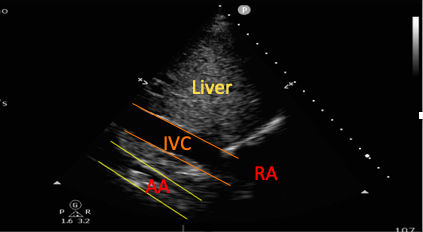
Figure 4. Subcostal view showing both the inferior vena cava and the abdominal aorta (AA).
RA=right atrium
How We Use the Subcostal Window
Below we describe several of the views obtainable from the SC window.
Subcostal Four-Chamber View (Coronal Plane)
The SC four-chamber view is achieved by placing the transducer under the patient’s xiphisternum, with the marker towards the patient’s left and the transducer held as flat against the patient’s abdominal wall as possible (Figure 5). Novice scanners commonly fail to obtain sufficient images secondary to insufficient pressure application or because the transducer is held too vertically.15
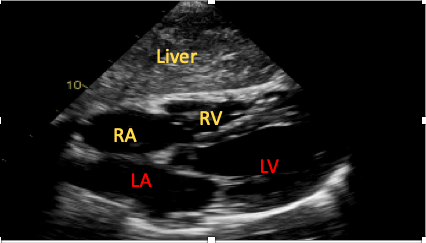
Figure 5. The subcostal four-chamber view.
RA=right atrium, RV=right ventricle, LA=left atrium, LV=left ventricle
Subcostal Five-Chamber View
This is achieved by first optimizing the SC four-chamber view, followed by cephalic (anterior) tilting of the transducer. This will gradually bring the LV outflow tract (LVOT) and aortic valve into view (Figure 6). It may be possible to apply spectral Doppler here; however, poor alignment limits its utility. 15
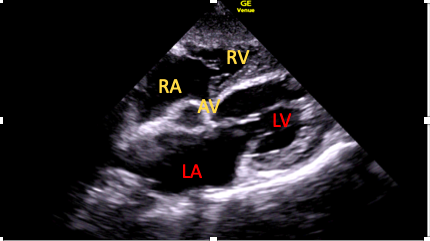
Figure 6. The subcostal five-chamber view.
AV=aortic valve
Subcostal Short-Axis Views
The SC four-chamber view should be obtained centered on the most basal aspect of the interventricular septum, and then a 70-90° anticlockwise rotation of the transducer should be performed. This view slices the heart in its short axis, with tilting of the transducer allowing for several different views to be obtained.
Subcostal Short-Axis Aortic View
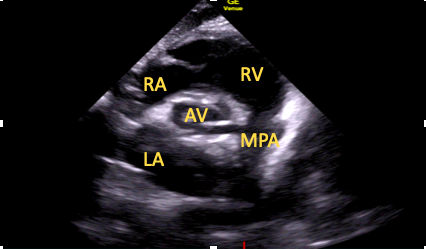
This should be the first short-axis view obtained using the above technique (Figure 7).15
Figure 7. Subcostal short-axis aortic view.
RA=right atrium, RV=right ventricle, AV=aortic valve LA=left atrium, MPA=main pulmonary artery
Subcostal Short-Axis Mitral Valve View
Tilting the transducer to point further towards the patient’s cardiac apex will bring the mitral valve leaflets into view (Figure 8).15
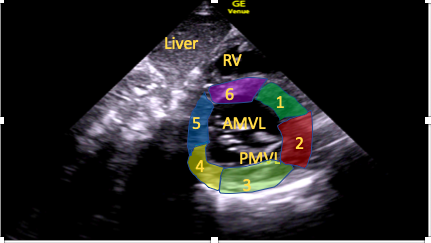
Figure 8. Subcostal short-axis mitral valve view.
RV=right ventricle, AMVL=anterior mitral valve leaflet, PMVL=posterior mitral valve leaflet; 1-6 are labelled segments of the LV wall: 1=anteroseptal, 2=anterior, 3=anterolateral, 4=inferolateral, 5=inferior, 6=inferoseptal
Subcostal Short-Axis Mid-Papillary Level
Further apical tilt will bring the papillary muscles into view, allowing for assessment of LV radial contraction (Figure 9).15
Figure 9. Subcostal short-axis mid-papillary muscle view.
RV=right ventricle, ALPM=anterolateral papillary muscle, PMPM=posteromedial papillary muscle
Subcostal Short-Axis Apical Level
Even further apical tilt will bring the LV apex into view (Figure 10), allowing for assessment of function and for the presence of thrombi.15
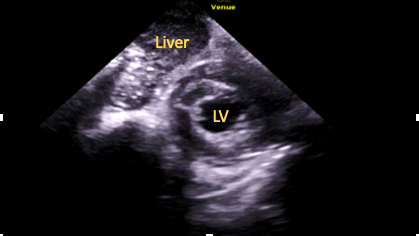
Figure 10. Subcostal
short-axis apical level view.
LV=left ventricle cavity
Subcostal Right Ventrical Outflow Tract View
Starting from the SC four-chamber view, cephalad tilting of the transducer is performed until the right ventricular outflow tract (RVOT) and pulmonary artery are visualized (Figure 11). Good cursor alignment means Doppler analysis of both the RVOT and pulmonary valve is often possible from this view.15
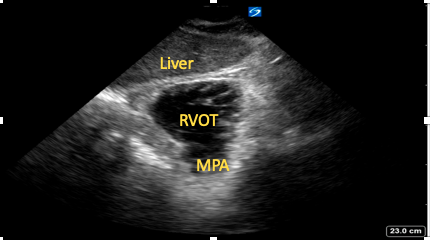
Figure 11. Subcostal
right ventricular outflow tract view.
RVOT=right ventricular outflow tract,
MPA=main pulmonary artery
Subcostal Inferior Vena Cava View
The SC four-chamber view should again be obtained, with the right atrium in the center of the screen and a 90° anti-clockwise rotation of the transducer performed. An element of tilting may then be required to bring the IVC into view and to visualize its entry into the right atrium (Figure 12). It is important not to mistake the IVC for the aorta or a hepatic vein. Techniques to avoid this include:
- Visualization of the IVC joining the right atrium
- Visualization of the hepatic vein as it joins the IVC
- Transverse (short axis) assessment of the IVC as it lies adjacent and to the right of the aorta
- The use of color flow and pulsed wave Doppler to assess flow pattern and direction.
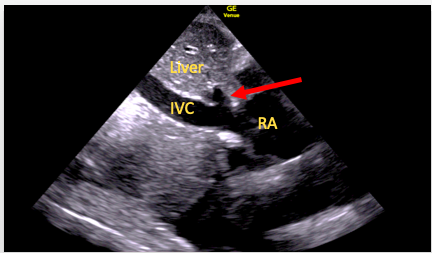
Figure 12. SC IVC view. Here the right hepatic vein (arrow) can be seen to join the IVC just before it enters the right atrium (RA)
SC Bicaval View
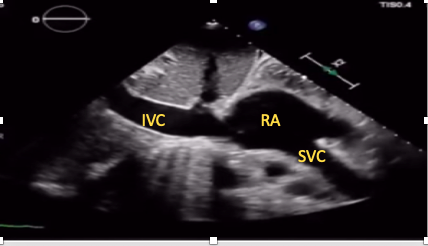
This view allows simultaneous visualization of the superior and IVC as they join the right atrium (Figure 13). It can be particularly useful for guidance of extracorporeal membrane oxygenation cannula and central venous cannula position confirmation.15,28,29 Tilting the transducer slightly laterally while in the IVC view brings the superior vena cava into view in most patients.
Figure 13. SC bicaval view.
IVC= inferior vena cava, SVC= superior vena cava, RA= right atrium
Subcostal Longitudinal Aortic View
The IVC longitudinal view should be gained, and then the transducer is tilted to the left until the aorta is visualized in long axis (Figure 14). This view allows for identification of aneurysms and dissections, as well as assisting extracorporeal membrane oxygenation cannula insertion.15,28,29
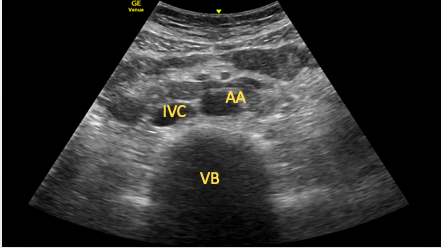
Figure 14. Longitudinal view of the abdominal aorta (outlined in yellow). The lumbar vertebrae are seen behind it (outlined in red).
Subcostal Short-Axis View of the Inferior Vena Cava and Aorta
The SC IVC or aortic short-axis view should be obtained and a 90° clockwise rotation performed. The transducer is then tilted to point caudally until the IVC and aorta are seen in transverse section (Figure 15). This view can be useful in identifying aortic dissections or aneurysms (as part of a full aortic scan).15

Figure 15. Transverse view of the aorta and inferior vena cava.
AA=abdominal aorta, IVC=inferior vena cava, VB=vertebral body
Pearls and Pitfalls
- As described above, the SC window can be extremely beneficial to the anesthesiologist. However, it is important to remain cognizant of a few of its limitations.
- The way in which the SC window slices the heart means cardiac chambers are often foreshortened, potentially resulting in underestimation of their dimensions and overestimation of their function.
- The oblique orientation to many cardiac structures may result in sub-optimal alignment of Doppler cursors, making accurate measurements challenging.
Conclusion
In the critical care and perioperative setting, transthoracic echocardiography is often limited by challenging cardiac views, particularly the parasternal and apical windows. The SC window often remains accessible in these patients and permits a significant cardiac assessment in multiple planes. It is thus vital that practitioners appreciate the full potential of the SC window as, for many patients, it may be the only available transthoracic window.
Olusegun Olusanya, Bsc(Hons), BM, MRCP(UK), FRCA, FFICM, EDIC, is a consultant in intensive care medicine at St Bartholomew’s Hospital in London, United Kingdom.
Luke Flower, MBChB, BSc, MRCP, is a honorary clinical research fellow at the William Harvey Research Institute of the Queen Mary University of London, and an anesthetic trainee at the Central London School of Anesthesia, both in London, United Kingdom.
Pradeep Madhivathanan, FRCP(Edin), FRCA, FFICM, EDIC, is a consultant in intensive care medicine and an anesthetist at Papworth Hospital in Cambridge, United Kingdom.
Matyas Andorka, MD, DESA, EDIC, is a consultant in anesthesia and intensive care medicine at Surrey and Sussex Healthcare NHS Trust in Redhill, United Kingdom.
References
- Vieillard-Baron A, Millington SJ, Sanfilippo F, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 2019;45:770-88. https://doi.org/10.1007/s00134-019-05604-2
- Millington SJ, Goffi A, Arntfield RT. Critical care echocardiography: a certification pathway for advanced users. L’échocardiographie aux soins intensifs: une voie vers la certification pour les utilisateurs avancés. Can J Anaesth 2018;65:345-9. https://doi.org/10.1007/s12630-018-1061-y
- Nanjayya VB, Orde S, Hilton A, et al. Levels of training in critical care echocardiography in adults: recommendations from the College of Intensive Care Medicine Ultrasound Special Interest Group. Australas J Ultrasound Med 209;22: 73-9. https://doi.org/10.1002/ajum.12127
- Mayo, PH. Training in critical care echocardiography. Ann Intensive Care 2011;1:36. https://doi.org/10.1186/2110-5820-1-36
- Singh K, Mayo P. Critical care echocardiography and outcomes in the critically ill. Curr Opin Crit Care 2018;24:316-21. https://doi.org/10.1097/MCC.0000000000000515
- British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470-83. https://doi.org/10.1136/thorax.58.6.470
- Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-1815. https://doi.org/10.1007/s00134-014-3525-z
- McLean AS. Echocardiography in shock management. Crit Care 2016;20:275. https://doi.org/10.1186/s13054-016-1401-7
- Flower L, Olusanya O, Madhivathanan PR. The use of point-of-care lung ultrasound and echocardiography in the management of coronavirus disease 2019 (COVID-19). J Cardiothorac Vasc Anesth 2020;S1053-0770(20)30430-4. https://doi.org/10.1053/j.jvca.2020.05.009
- Lazzeri C, Cianchi G, Bonizzoli M, et al. The potential role and limitations of echocardiography in acute respiratory distress syndrome. Ther Adv Respir Dis 2016;10:136-48. https://doi.org/10.1177/1753465815621251
- Gupta NK, Agrawal RK, Srivastav AB, et al. Echocardiographic evaluation of heart in chronic obstructive pulmonary disease patient and its co-relation with the severity of disease. Lung India 2011;28:105–9.
- Flower L, Olusanya O, Madhivathanan PR. The use of critical care echocardiography in peri-arrest and cardiac arrest scenarios: pros, cons and what the future holds. [published online first]. J Intensive Care Soc. June 2020. https://doi.org/ 10.1177/1751143720936998 .
- Wharton G, Steeds R, Allen J, et al. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract 2015;2:G9-24. https://doi.org/10.1530/ERP-14-0079
- British Society of Echocardiography. Critical care echocardiography accreditation [Internet]. [cited 2020 June 25]. Available from: https://www.bsecho.org/Public/Accreditation/Accreditation-subpages/Personal-accreditation-subpages/Critical-care-echocardiography-accreditation.aspx
- Flower L, Madhivathanan PR, Andorka M, et al. Getting the most from the subcostal view: the rescue window for intensivists. J Crit Care 2021;63:202-10. https://doi.org/10.1016/j.jcrc.2020.09.003
- Pérez-Casares A, Cesar S, Brunet-Garcia L, et al. Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Front Pediatr 2017;5:79. https://doi.org/10.3389/fped.2017.00079
- Cruz I, Stuart B, Caldeira D, et al. Controlled pericardiocentesis in patients with cardiac tamponade complicating aortic dissection: experience of a centre without cardiothoracic surgery. Eur Heart J Acute Cardiovasc Care 2015;4:124-8. https://doi.org/10.1177/2048872614549737
- Drummond JB, Seward JB, Tsang TS, et al. Outpatient two-dimensional echocardiography-guided pericardiocentesis. J Am Soc Echocardiogr 1998;11:433-5. https://doi.org/10.1016/s0894-7317(98)70022-7
- Ainsworth CD, Salehian O. Echo-guided pericardiocentesis: let the bubbles show the way. Circulation 2011;123:e210-e211. https://doi.org/10.1161/CIRCULATIONAHA.110.005512
- Martin SS, Shapiro EP, Mukherjee M. Atrial septal defects - clinical manifestations, echo assessment, and intervention. Clin Med Insights Cardiol 2015;8:93-8. https://doi.org/10.4137/CMC.S15715
- Silvestry FE, Cohen MS, Armsby LB, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr 2015;28:910-58. https://doi.org/10.1016/j.echo.2015.05.015
- Modin D, Møgelvang R, Andersen DM, et al. Right ventricular function evaluated by tricuspid annular plane systolic excursion predicts cardiovascular death in the general population. J Am Heart Assoc 2019;8:e012197. https://doi.org/10.1161/JAHA.119.012197
- Díaz-Gómez JL, Alvarez AB, Danaraj JJ, et al. A novel semiquantitative assessment of right ventricular systolic function with a modified subcostal echocardiographic view. Echocardiography 2017;34:44–52. https://doi.org/10.1111/echo.13400
- Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med 2004;30:1740-6. https://doi.org/10.1007/s00134-004-2259-8
- Hartshorne TC, McCollum CN, Earnshaw JJ, et al. Ultrasound measurement of aortic diameter in a national screening programme. Eur J Vasc Endovasc Surg 2011;42:195-9. https://doi.org/10.1016/j.ejvs.2011.02.030
- Costantino TG, Bruno EC, Handly N, et al. Accuracy of emergency medicine ultrasound in the evaluation of abdominal aortic aneurysm. J Emerg Med 2005;29:455-60. https://doi.org/10.1016/j.jemermed.2005.02.016
- Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ulstraound: development of the venous excess ultrasound grading system. Ultrasound J 2020;12:16. https://doi.org/10.1186/s13089-020-00163-w
- Douflé G, Roscoe A, Billia F, et al. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015;19:326. https://doi.org/10.1186/s13054-015-1042-2
- Viau-Lapointe J, Douflé G. Transthoracic view of extracorporeal membrane oxygenation cannulae. Am J Respir Crit Care Med 2019;199:e39-e40. https://doi.org/10.1164/rccm.201806-1153IM