How I Do It: Celiac Plexus Block
Cite as: Neal K. How I do it: celiac plexus block. ASRA Pain Medicine News 2022;47. https://doi.org/10.52211/asra020123.006
Anatomy
The celiac plexus resides anterior to the T12-L2 vertebral body and is wrapped around the aorta at the level of the celiac artery. It carries visceral sympathetic, parasympathetic, and sensory fibers to and from a variety of organs, including the liver, gallbladder, stomach, pancreas, distal esophagus, diaphragm, suprarenal glands, kidneys, ovaries/testes, small bowel, and colon up to the splenic flexure. Given its sunburst appearance in gross dissection, the celiac plexus has been described as the “solar plexus.” It communicates with the spinal cord via the greater, lesser, and least splanchnic nerves (T5-T12).1
A celiac plexus block is commonly performed for diagnosis and therapy.
Patient Selection
As with most therapies, the key to procedural success begins with proper patient selection. Celiac plexus blocks are classically discussed as a treatment option for visceral abdominal pain related to pancreatic cancer. Other common anatomic distributions that may respond to celiac plexus intervention include pain related to pathology of the liver, gallbladder, stomach, and upper small intestine.1 These are often cancerous in nature but can be non-cancerous as well, such as chronic pancreatitis. Discerning whether a patient’s pain is visceral or within the territory of the celiac plexus can be difficult. In these circumstances, a celiac plexus block is commonly performed for diagnosis and therapy.
Choosing the Approach
There are two common approaches to the celiac plexus: retrocrural and transgastric. To determine which approach is best for the patient, I recommend reviewing the patient’s spiral imaging, paying particular attention to approach angles, depth, and needle path. The following factors are important in determining whether a retrocrural approach should be pursued:
- Depth
- Angle of approach (kidney blocking approach)
- Mass blocking approach
- Lymphadenopathy
- Pleura or vascular abnormalities preventing a safe needle pass.
If a retrocrural approach is prohibited, I recommend discussing the transgastric approach with gastroenterology (GI) team. If the GI team cannot perform the procedure or if the patient prefers to avoid general anesthesia and esophagogastroduodenoscopy, I recommend evaluation for a splanchnic block.
Patient convenience also should be considered, particularly in patients with cancer. Many of these patients have several upper GI endoscopies during their workup and care. If the patient already has an endoscopy planned with gastroenterology, the GI team may be able to perform the block and neurolysis at the same time as the other procedure.
Absolute contraindications to a retrocrural celiac block include
- Patient refusal
- Inability to access the appropriate injection site.
Relative contraindications include
- Infection overlying the injection site
- Intra-abdominal infection
- Hypotension
- Inability to stop anticoagulants for the appropriate amount of time
- Thrombocytopenia
- Mass effect
- Abdominal aortic aneurysm
- Presence of a bowel obstruction.2
Choice of Treatment
It is important to determine patient candidacy for a local anesthetic block versus alcohol neurolysis. This is an individual decision for each patient based on diagnosis, prognosis, blood thinner status/risk, social circumstances, and, ultimately, patient preference. Patients should engage in a proper informed consent and discussion with the physician to ensure appropriate decision making by the physician and patient.
Sedation
Minimal to moderate sedation enables communication with the patient and subsequent intra-operative monitoring. This allows patient feedback if a nerve root is contacted and allows neurologic evaluation during alcohol neurolysis. Even if a patient requests no sedation, all patients are required to have IV access, and, unless contraindicated, a crystalloid fluid bolus is administered during the procedure to offset possible orthostatic hypotension.
Retrocrural Celiac Plexus Block
The first procedural step is to identify the L1 vertebral body. Though this may seem easy, the estimated rate of lumbosacral transitional vertebrae is 9.9%.3 Additionally, up to 8% of children have only 11 ribs, and the presence of a lumbar rib has been quoted as high as 16%.4,5 Spiral imaging can be used to evaluate for abnormal lumbar anatomy preoperatively. After correlating imaging, the inferior endplate of L1 should be aligned and the level marked with an 18 g needle. Then, adjust the fluoroscopy laterally to 20-30 degrees or until the lateral edge of the transverse process is just posterior to the anterior vertebral body wall. The target is the superior or inferior lateral edge of the transverse process. Either approach is acceptable, depending on patient anatomy, but I most often use the inferior lateral edge to remain as far from pleura as possible. This will place the target at the inferior 1/3 of the vertebral body. If the disc is anticipated to be contacted with this view, I use the superior lateral edge or tilt inferiorly until the disc is no longer predicted to be contacted.
Ultimately, the goal of the needle approach is to be lateral enough to the nerve root such that it has already descended and the needle will not make contact. Again, this is a good reason to use minimal sedation, allowing the patient to provide feedback during needle advancement.
Injection Technique
Next, 1% lidocaine is placed subdermally, and a 5- or 7-inch 22-g Quinke spinal needle is used to approach the vertebral body in coaxial fluoroscopic view. A moderate bend on the tip of the needle assists with “walking the needle” around the vertebral body. It is crucial to keep the needle coaxial given the depth and adjustments needed after contacting the vertebral body. The needle insertion point is 1-2 mm posterior to the anterior border of the vertebral body, utilizing it as a backstop, knowing the bend in the needle will allow further advancement in the future.
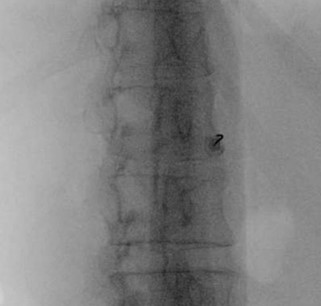
Figure 1. Coaxial approach at the L1 vertebral body, with a 25-degree angle, which was consistent with the angle measured on computerized tomography of the abdomen.
Once the vertebral body is contacted, needle advancement is stopped until it is in lateral view. Prior to proceeding to lateral view, the contralateral side is conducted using the technique described above. Once the second needle contacts the vertebral body, fluoroscopy is moved laterally. To distinguish the two needles in lateral, one needle’s bend is turned superior and one needle’s bend inferior. In the lateral view, the needle is adjusted until the tip is just anterior to the vertebral body, by not more than 1 mm. This often requires “walking the needle” around the vertebral body by rotating the tip slightly lateral and advancing prior to turning it back medial, maintaining close contact with the vertebral body throughout. During the final adjustments, if there is any change in resistance, pause and aspirate for heme. Once the needle is at appropriate depth, 2-3 cc of contrast are injected with a live fluoroscopic exposure in the lateral view for each needle. This allows visualization of vascular uptake, which cannot be appreciated with single-shot images. Ideal contrast spread is anterior to the vertebral body moving in a superior and inferior plane without vascular uptake. Contralateral contrast injection should darken the contrast image already present. If possible, adjust each needle to the final target location prior to injecting contrast. Once contrast is placed, it will partially obscure your view of the contralateral needle tip.
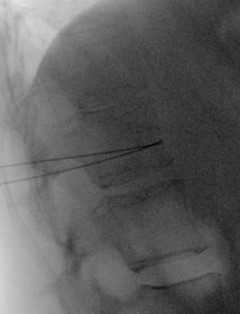
Figure 2. Lateral radiograph with bilateral needles fully advanced to the anterior vertebral body prior to injection of contrast. One needle is bent upward, and one needle is bent downward.
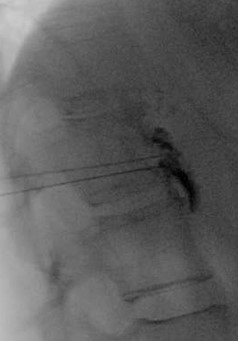
Figure 3. Lateral radiograph with ideal contrast spread along anterior vertebral body. No contrast is noted intravascularly.
After lateral contrast confirmation of each needle, an anteroposterior view will be obtained. Ideal needle tip placement will be near the medial border of the pedicle shadow. Following negative aspiration, a small amount of contrast is injected into each needle to evaluate spread under live fluoroscopy. Ideal contrast spread remains within the radiographic confines of the vertebral body. If adjustment is needed, these changes should be conducted in the lateral view.
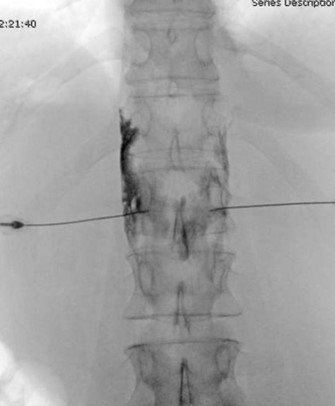
Figure 4. Anteroposterior (AP) radiograph demonstrating appropriate final needle placement at the medial border of the pedicle. Contrast in AP radiograph demonstrates spread within the confines of the vertebral body. The left-sided needle first had spread along the lateral vertebral body and was adjusted anteriorly for improved contrast spread prior to this image.
Injectate
Once both needles are in the appropriate location and confirmed with contrast, a test dose of 3 mL of 1% lidocaine with 1:400 k epinephrine is placed into the first needle. Then the patient’s vitals are monitored for two minutes; a 20% increase in heart rate is indicative of intravascular injection. After the two-minute negative test dose on the first side, a test dose is administered in the contralateral side. In addition to testing for intravascular injection not detected with contrast, this also provides rapid onset local anesthetic to the plexus if alcohol neurolysis is being performed. I find it helpful to use extension catheters on each needle to take tension off the needles, help prevent inadvertent needle movement during aspiration and injection, and reduce radiation exposure to the proceduralists hands.
For local anesthetic injection only, use 15 cc of 0.25% bupivacaine with 2 mg dexamethasone in each needle, without reaching a maximum toxic dose. For alcohol neurolysis, first use 8-10 mL of 98% ethanol on each side. This is injected in alternating 2 mL aliquots every 2 minutes with quadriceps strength checked before each additional aliquot. After completion of the neurolysis, 6-8 mL of 0.25% bupivacaine is injected into each side at the plexus, followed by 1-2 mL during needle withdrawal.
Postanesthesia Care Unit
The patient’s 1 L crystalloid bolus is completed postoperatively if it was not completed during the procedure. The patient is encouraged to avoid rapid position changes and is monitored for orthostatic hypotension. Patients may feel intoxicated from the ethanol, and they are warned not to drink or operate machinery for 24 hours afterwards. A lower extremity neurologic exam is conducted and documented before and after the procedure. Patients can be discharged home if no procedural complications are noted. Red-flag symptoms and return-to-care instructions are given prior to discharge.
Conclusion
In properly selected patients, the celiac plexus block and neurolysis remain a cornerstone of visceral abdominal pain management, particularly in the cancer population.
Procedural Pearls
- Proper patient selection is key to post-procedural success.
- L1 misidentification is a potential procedural error and may lead to suboptimal pain relief.
- Advancing each needle to the vertebral body in coaxial view prior to moving to lateral can improve efficiency.
- After initial vertebral body contact, all further adjustments should be conducted in lateral.
- Utilizing a test dose with epinephrine can help confirm positioning as well as provide local anesthetic for alcohol neurolysis.
- Pre, intra, and postoperative neurologic evaluation with documentation is an important safety measure.
- Intra-operative fluid bolus may assist in reducing post-procedural hypotension.

Kevin Neal, MD, is an assistant professor in the department of Anesthesiology and Pain Management at the University of Texas Southwestern in Dallas, where he serves on the cancer pain management and general and regional anesthesiology teams.
References
- Gofeld M, Shankar, H. Peripheral and visceral sympathetic blocks. In Benzon HT, et al. (Eds.). Practical Management of Pain. 5th ed, pp. 759-63. Philadelphia, PA: Elsevier Mosby, 2014.
- Kambadakone A, Thabet A, Gervais DA, et al. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. RadioGraphics 2011;31:1599-621. https://doi.org/10.1148/rg.316115526.
- French HD, Somasundaram AJ, Schaefer NR, et al. Lumbosacral transitional vertebrae and its prevalence in the Australian population. Global Spine J 2014;4(4):229-32. https://doi.org/10.1055/s-0034-1387808.
- Khodair SA, Hassanen OA. Abnormalities of fetal rib number and associated fetal anomalies using three dimensional ultrasonography. Egypt J Radiol Nucl Med 2014; 45: 689-94. https://doi.org/10.1016/J.EJRNM.2014.03.009.
- Chengetanai S, Nchabeleng EK, Bacci N, et al. Supernumerary lumbar ribs: a rare occurrence on an adult African male skeleton. Anat Cell Biol 2017;50(2):155-8. https://doi.org/10.5115/acb.2017.50.2.155.
Leave a commentOrder by
Newest on top Oldest on top