How I Do It: Occipital Nerve Stimulator Implant Procedure Guidance
Cite as: Nurre A, Ali H, Sana M, et al. How I do it: occipital nerve stimulator implant procedure guidance. ASRA Pain Medicine News 2023;48. https://doi.org/10.52211/asra080123.009.
Introduction
This guide is designed to present the preoperative considerations, procedure planning, and postoperative considerations for the placement of an occipital nerve stimulator. We will discuss the necessary equipment, C-arm positioning, sedation, patient preparation, neuraxial and surgical technique, and stimulator testing.
Occipital nerve stimulation is a form of peripheral neuromodulation therapy and involves an implantable device composed of an electrode and pulse generator. The lead is placed into the subcutaneous tissues innervated by the greater and lesser occipital nerves, and the pulse generator is implanted into a subcutaneous pocket in the chest, abdomen, or back. A permanent device is implanted under anesthesia, following a successful trial during which time leads with an external power generator are used. This trial can last between four to seven days. A pain diary is kept during the trial period and significant improvements in pain (more than 50%) and quality of life must be reported. Therapy and surgical implantation are primarily aimed at treating occipital neuralgia, headaches, and craniofacial pain, but they have been used to treat other diseases. New and existing data can demonstrate the benefit of this device.
These studies include the Occipital Nerve Stimulation for the Treatment of Chronic Migraine Headache trial (2011), during which 39% of patients reported having least a 50% reduction in headache days per month or a three-point improvement in pain intensity at 3 months.1 A randomized control trial published in 2012 demonstrated that nerve stimulation for migraines reduced associated pain and related disability by 30%.2
Although relatively rare, complications from the placement of occipital nerve stimulators are possible.
Although relatively rare, complications from the placement of occipital nerve stimulators are possible. Complications include, but are not limited to, lead migration, device shock, muscle spasms, infection, wound dehiscence, bleeding, and nerve damage. Of these, the most commonly reported complication is lead migration (incidence up to 10%), which is often attributed to trauma.3 Lead migration can typically be prevented by avoiding excessive neck movement. Infection rates as high as 4% have been documented.1 Infection is prevented with adherence to appropriate sterile surgical technique and prophylactic antibiotics when indicated.
Pre-procedural Care and Planning
Prior to implantation, a trial is performed in which leads are placed under the skin and connected to an external battery. The trial is performed under sedation, and the patient is discharged the same day. Afterward, the patient tries the therapy for 7 days and keeps a detailed pain diary. To mitigate complications, all patients are swabbed to rule out methicillin-resistant staphylococcus aureus colonization and provided pre-implantation antibiotics to eliminate colonization. The occipital region is trimmed of hair, and the sites of lead insertion and IPG implantation are clearly marked. The procedure is performed under sterile surgical technique to reduce the chance of infection.
Equipment
The following is a comprehensive but not complete listing of equipment that will be required. Prior to beginning the procedure, leads of the appropriate length need to be in the room along with regular and curved coude epidural needles (authors’ preference) (Figure 1). The operating room bed should be compatible with C-Arm use over the head, thoracic, and lumbar spine (Figure 2). The bed should also have an adjustable head piece to improve neck flexion while the patient is in the prone position. Lead aprons need to be available for all participants.

Figure 1. Curved coude epidural needle.

Figure 2. Bed compatible with C-arm.
Medications
Medications for the procedure will include 1% plain lidocaine, 0.25% bupivacaine with 1;200,000 epinephrine, vancomycin 1 gram powder, and normal saline for irrigation.
Anesthesia
Moderate to deep sedation can be administered with localized lidocaine or other anesthetics.
Positioning
The patient will usually be positioned prone on the operating table depending on the incision entry point, and the pillow will be positioned under the abdomen, pelvis, and shoulders. (Figure 3) Position the neck over the break point between the bed and headpiece and neck flexion is essential for better insertion of needles. The head can be positioned over the foam cradle. (Figure 4) The head and shoulders can then be stabilized with three-inch silk tape. (Figure 5) The arms are tucked at the patient’s side, and arm boards are used for patient comfort. Monitoring wires should be placed to avoid being under the head and neck area, and all pressure points should be evaluated and padded.
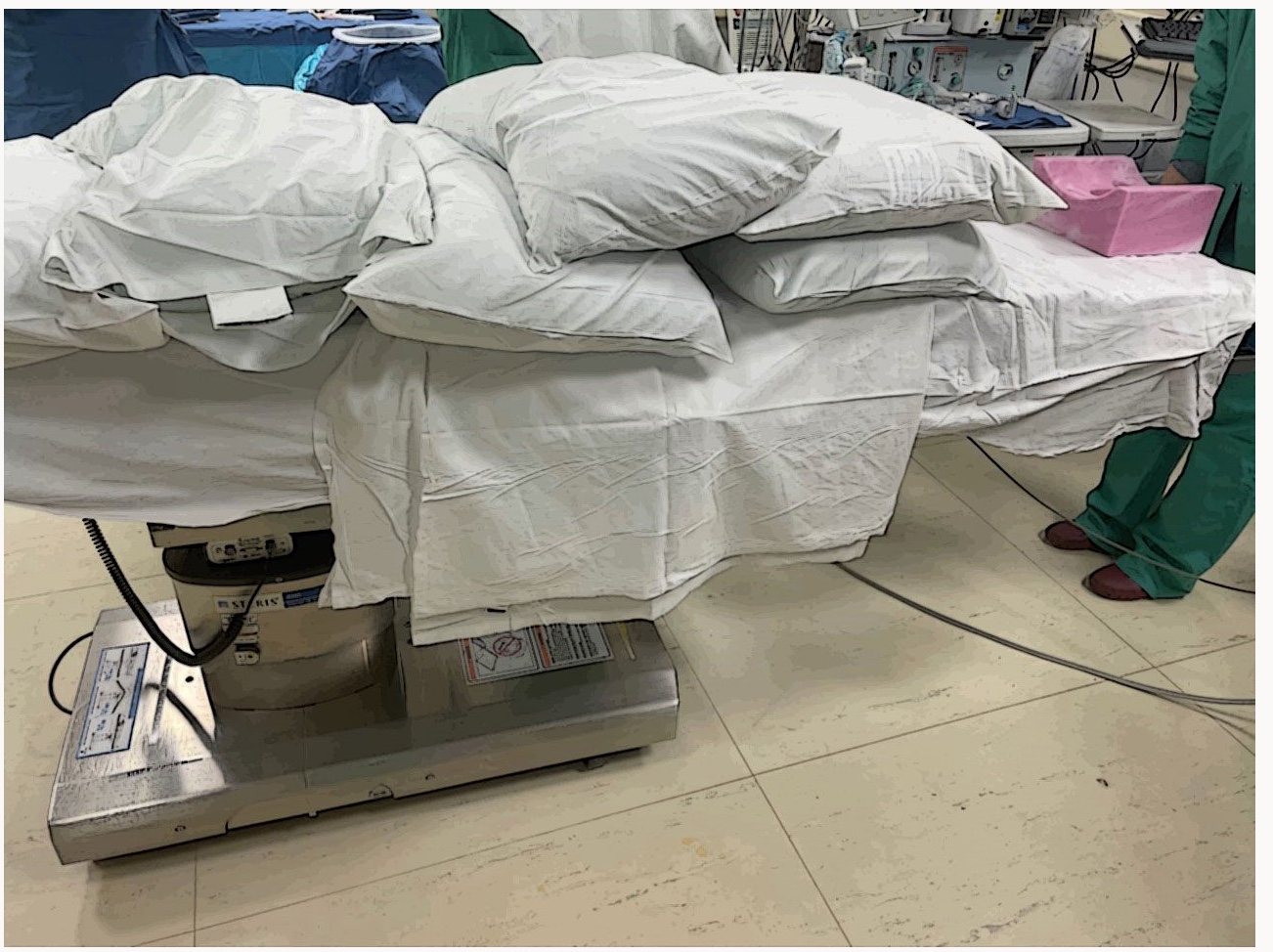
Figure 3. Pillow placement is under the abdomen, pelvis, and shoulders.

Figure 4. Foam head cradle.
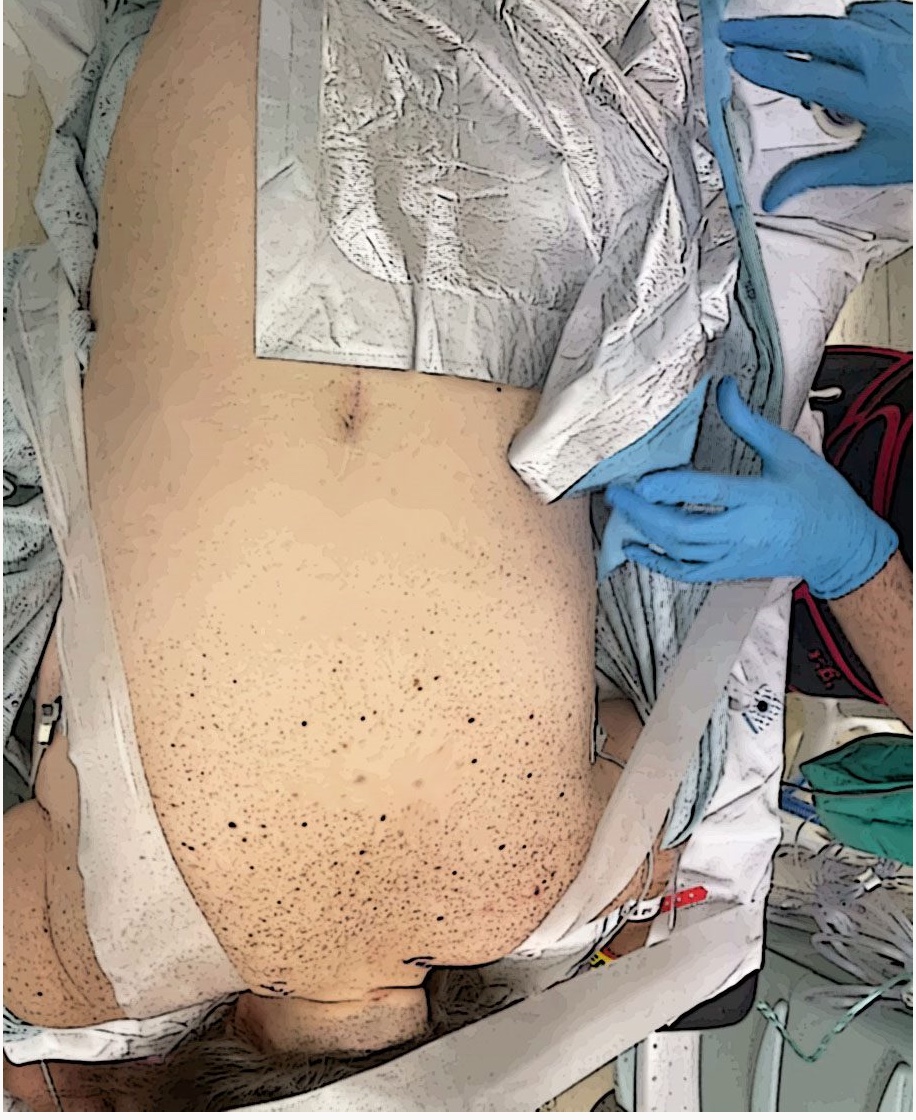
Figure 5. Stabilizing the surgical field with silk tape.
Incision Site Preparation
The occipital region should be trimmed with clippers. Six 10x10 plastic drapes will be placed over patient as seen in (Figure 6). Sterile blue towels are then placed over plastic drapes as shown in (Figure 7). In addition, four separate surgical drapes without single holes and two Ioban antimicrobial drapes are placed as shown in (Figure 8).

Figure 6. Placement of plastic drapes.

Figure 7. Blue towels.
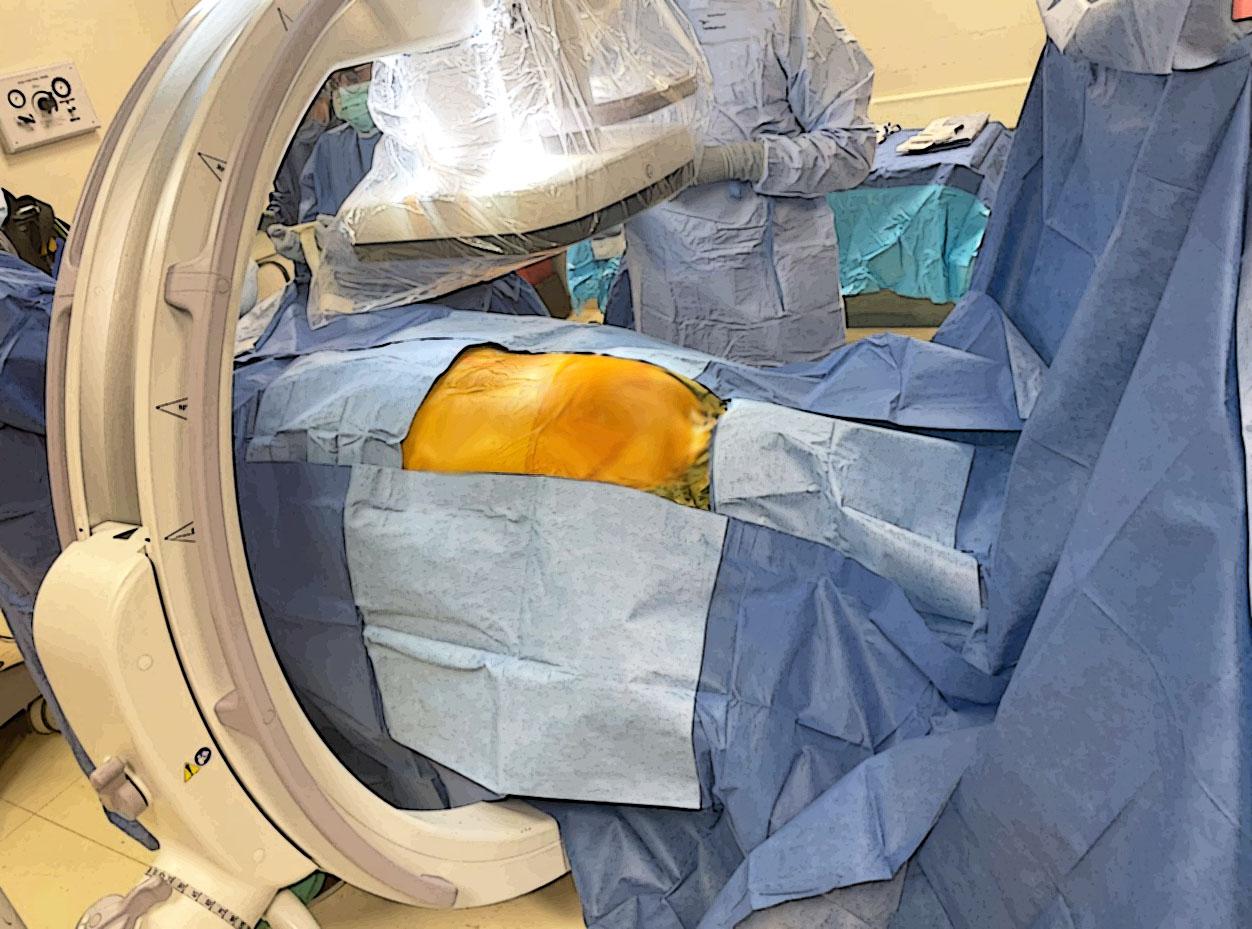
Figure 8. Complete setup with surgical and Ioban drapes.

Figure 9. Fluoroscopic imaging confirming electrode placement.
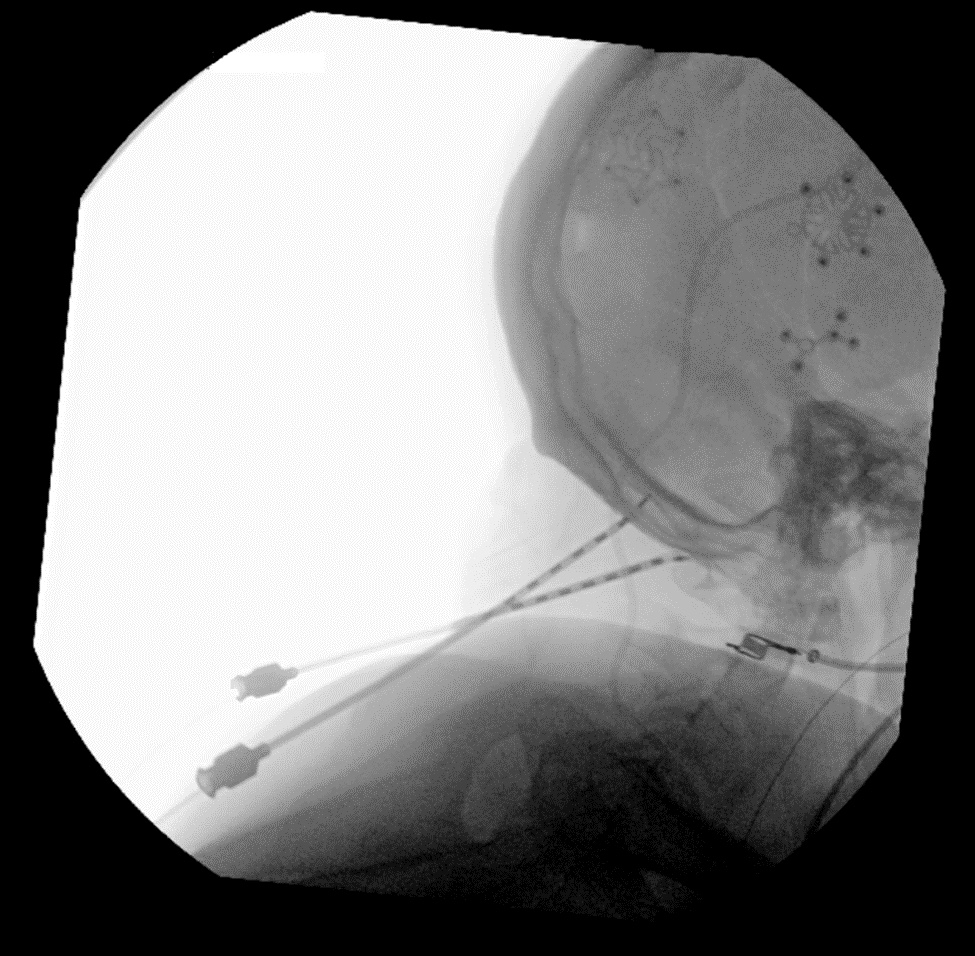
Figure 10. Fluoroscopic imaging confirming electrode placement.
Procedure
After the patient is brought to the operating room and placed in the prone position, these steps are followed to place an occipital nerve stimulator:
- She is prepped and draped in the usual sterile fashion, using chlorhexidine and ChloraPrep three times.
- With fluoroscopic guidance, the location of the desired site for placement of the percutaneous lead is identified, and local anesthetic consisting of 1% Lidocaine and 0.25% bupivacaine with epinephrine is injected subcutaneously.
- An approximately 4-centimeter longitudinal incision is made in the midline of the neck over C2-C4. The incision is dissected to the supraspinous ligament. Hemostasis is established, and a self-retaining retractor is used to allow adequate visualization.
- A 14-gauge Tuohy needle is then introduced using the paraspinous approach under fluoroscopic guidance and advanced in between muscle planes laterally from the midline at C2-C3 towards the mastoid process laterally on both sides.
- Once the electrodes are placed into the needle, the needle is removed using intermittent fluoroscopy to ensure that the electrodes remain in the desired anatomic location (Figures 9 and 10).
- The screen cable connector is then joined to the lead, and the screen cable is then connected to the screener. A test stimulation is performed, and adequate coverage of the painful areas is ensured. The screen cable is removed from the lead.
- The leads are then anchored to the supraspinous ligaments with 2-0 silk suture.
- A pocket site is identified based on the preoperative discussion with the patient. Local anesthetic is administered in the region, and the incision is made. A subcutaneous pocket is then created using a blunt dissection technique for placement of the pulse generator.
- Then tunneling is done between the lead and the pocket site after the administration of additional local anesthetic subcutaneously. The tunneling tool has a wedged tip and is introduced subcutaneously from the lead incision and guided to the pocket.
- The leads are inserted into the tunneling catheter and advanced to the pocket site. The leads are then inserted into the pulse generator, and the screw is tightened with the hex wrench.
- The generator is then introduced into the pocket and remote tested to ensure adequate impedance. All surgical wounds and incisions are then irrigated with saline solution; after that the wounds are closed.
- A sterile dressing is applied, and no specimens are collected.
Typical implant locations include the upper buttock, which facilitates single-stage electrode and generator placement in the prone position; the abdomen, which is performed with the patient in the lateral position, and the upper chest in the lateral or supine position.
Post Procedural Care
The patient should be monitored in post-operative care per institution guidelines and will be discharged home with short-course oral antibiotics.
Conclusions
Occipital nerve stimulation is a form of neuromodulation therapy that is a promising treatment modality for chronic intractable migraines. Our procedure guide for occipital nerve stimulator implantation presents a detailed step-by-step guide with images to serve as a resource for providers interested in providing this treatment for their patients.
Alexander Nurre, MD, is an is an anesthesiologist at Ochsner Health System in New Orleans, Louisiana.
Haider Ali, MD, is an anesthesiologist at Ochsner Health System in New Orleans, Louisiana.
Maddassir Sana, MBBS, is an anesthesiologist at Our Lady of the Lake Regional Medical Center in Baton Rouge, Louisiana.
Maged Guirguis, MD, is an anesthesiologist at Ochsner Health System in New Orleans, Louisiana.
Yashar Eshraghi, MD, is the program director for the pain medicine fellowship training program at Ochsner Health System in New Orleans, Louisiana.
References
- Saper JR, Dodick DW, Silberstein SD, et al. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 2011;31(3):271-85. https://doi.org/10.1177/0333102410381142
- Serra G, Marchioretto F. Occipital nerve stimulation for chronic migraine: a randomized trial. Pain Physician2012;15(3):245-53.
- Sakharpe AK, Cascella M. Occipital nerve stimulation. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- Allen B, McEvoy M. Competency assessment in regional anesthesia: quantity today, quality tomorrow. Reg Anesth Pain Med 2017;42(4):429-31. https://doi.org/10.1097/AAP.0000000000000626
- Warm E, Rosenblum M, et al. A Guidebook for Implementing and Changing Assessment in the Milestones Era. Chicago, IL: Accreditation Council for Graduate Medical Education; 2020.
- Chuan A, Forrest K, et al. Competency-based assessment tools in regional anesthesia: a narrative review. Br J Anaesth2018;120(2):264-73. https://doi.org/10.1016/j.bja.2017.09.007
- Chuan A, McLeod G. Tools to assess regional skills within a competency-based curriculum. ASRA Pain Medicine News. 2022;47. https://doi.org/10.52211/asra080122.034
- Woodworth G, Barrington M, et al. Anesthesia residency training in regional anesthesiology and acute pain medicine: a competency-based model curriculum. Reg Anesth Pain Med 2020;45(8):660-7. https://doi.org/10.1136/rapm-2020-101480

