TRPV1 Modulation: A “Spicy” Approach to Pain Relief
Cite as: Atawala N, Ambai VT, Kalava A. TRPV1 modulation: a “spicy” approach to pain relief. ASRA Pain Medicine News 2023;48. https://doi.org/10.52211/asra110123.006.
Introduction
Transient receptor potential vanilloid 1 (TRPV1) is a transmembrane cation channel protein responsible for the conduction of thermal and chemical stimuli to the central nervous system, primarily to relay the sensation of pain. Topical doses of capsaicin, the active chemical component of hot peppers, have been used to induce desensitization of this channel and relieve pain for a wide range of conditions, including post-herpetic neuralgia (PHN) and diabetic peripheral neuropathy (DPN).1 Around the same time that the 2021 Nobel Prize in Physiology or Medicine was awarded to the discoverers of TRPV1, favorable results from a clinical trial of a lipophilic injectable capsaicin pro-drug known as vocacapsaicin were published.2 These recent events have renewed interest in capsaicin analogs and TRPV1 antagonists as non-opioid alternatives for treating pain ranging from osteoarthritis (OA) to post-surgical pain with recent studies demonstrating significant reductions in pain at rest, pain with ambulation, and opioid consumption.2
History and Function
First identified in 1997, TRPV1 is an ion channel protein crucial to the transduction of both thermal and chemical stimuli.3 This channel was isolated to identify how capsaicin, the “hot” chemical component in certain peppers, produces a burning sensation when consumed. It is known to be a non-selective cation channel that functions as a nociceptor within the peripheral nervous system and is highly expressed in ganglia of the dorsal root, trigeminal, and vagus nerve.4 Initial exploratory studies of the physiology of TRPV1 revealed an activation threshold greater than 40°C in response to heat, prompting further investigation into stimuli able to generate a sensation of thermal pain through this channel.5 In addition to elevated temperatures, TRPV1 is known to depolarize in response to pH < 6.5, osmolarity changes, arachidonic acid metabolites, and endocannabinoids.4
The core of the TRPV1 receptor is an ion-conducting pore activated by capsaicin, heat, protons, and other ligands that induce conformational change, allowing for the influx of primarily calcium ions to generate a nociceptive action potential (Figure 1A). 1 Once depolarization occurs, TRPV1 undergoes a conformational change that increases the likelihood of further activation by heat, protons, and capsaicin. The induction of this hyperexcitable state plays a critical role in the propagation of tissue inflammation and consequent hyperalgesia.1 Additionally, a variety of pro-inflammatory agents, particularly protein kinase C and nerve growth factor, have been found to contribute to TRPV1-mediated hyperalgesia and allodynia by lowering the channel’s activation threshold and enhancing sensitivity to agonist molecules.4,6
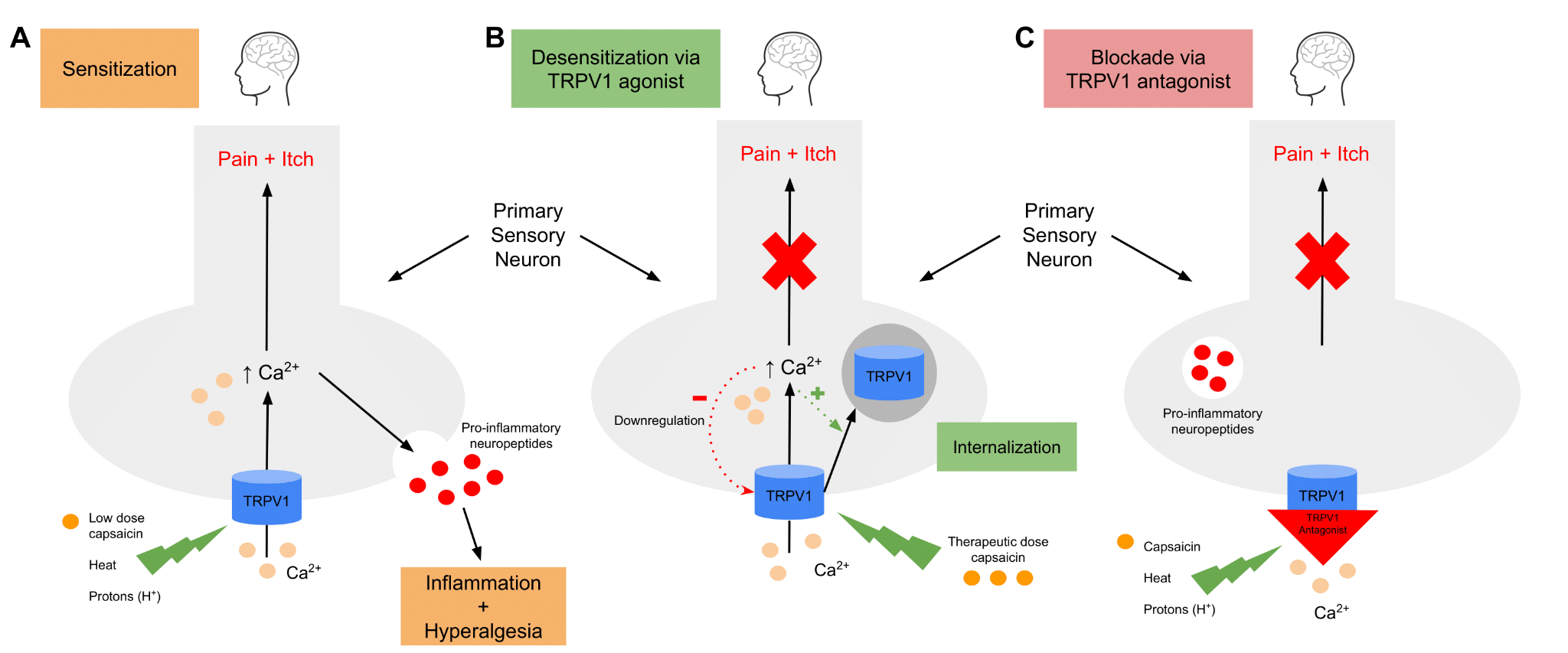
Figure 1.
A: Agonists, including capsaicin, heat, and protons, induce a conformational change in the TRPV1 channel, allowing a calcium-dependent depolarization to occur. Not only does this transmit a pain signal to the brain, but it also leads to the release of inflammatory neuropeptides, which further contribute to the sensation of hyperalgesia.
B: Continuous stimulation of TRPV1 by capsaicin induces a process of endocytosis, in which TRPV1 is internalized and trafficked to the lysosome for degradation. This renders the neuron temporarily unresponsive to stimulation by agonistic molecules, resulting in a state of analgesia.
C: Non-competitive TRPV1 antagonists function by blocking the aqueous pore of the channel, therefore preventing calcium influx when interacting with agonists.
The therapeutic benefit of capsaicin became more apparent after repeated applications. Studies found that repeated capsaicin application induced a calcium-dependent desensitization of TRPV1, making the channel insensitive to capsaicin and other noxious stimuli for a prolonged period of time (Figure 1B).4 This mechanism has been proposed to involve dose and time-dependent channel activation with subsequent calcium entry into the cell resulting in downregulation and clathrin-independent endocytosis of the TRPV1 channel.6 Once internalized, TRPV1 is trafficked to the lysosome for degradation, thereby interrupting nociceptive signal transmission. This process has been exhibited in dorsal root ganglion (DRG) neurons as well as other TRPV1-expressing cells.6 The discovery of the desensitization and downregulation of TRPV1 in response to repeated capsaicin application laid the foundation for the exploration of therapeutic options involving the use of TRPV1 modulators to treat chronic pain. Analgesia may also be induced via the use of noncompetitive TRPV1 antagonists, which operate by blocking calcium influx through the aqueous channel of the receptor (Figure 1C).7
Current Use and Future Potential
Capsaicin is currently approved for delivery via patches or a variety of liniments (creams, gels, ointments, lotions, liquids, etc.). Over-the-counter formulations reach concentrations of 0.1% and are labeled for use in treating muscle/joint pain, but are also used off-label for diabetic neuropathy, psoriatic pain, and as counterirritants to pruritus.8 In 2009, a new formulation of capsaicin with a concentration of 8% was approved for prescription use for PHN and DPN.9 This high dose prescription is administered by a healthcare professional and requires pretreatment of the area with a topical anesthetic. Multiple patches can be applied for 30-60-minute treatments, which can be repeated every 3 months as needed.
The undesirable side effect of acute burning pain upon topical application is avoided with direct intra-articular injections or injection of solutions during surgery while patients are anesthetized.
Given the limited penetration of topical applications and the potential benefit of capsaicin as a non-opioid modality to reversibly disrupt pain sensation, newer studies are investigating injectable capsaicin and capsaicin analogues to target the TRPV1 receptors responsible for transmitting nociceptive signals.9 The undesirable side effect of acute burning pain upon topical application is avoided with direct intra-articular injections or injection of solutions during surgery while patients are anesthetized. The injectable solutions require lower doses of medication compared to topical capsaicin and are being studied for use as long-acting, one-time injections. Current conditions with promising results after injectable capsaicin treatment include OA-related pain as well as post-surgical pain. 4
A proprietary injectable trans-capsaicin formulation with an elimination half-life of less than 4 hours was evaluated in the TRIUMPH study (ClinicalTrials.gov Identifier: NCT02558439). This was a phase 2b, multi-center, randomized (2:1:2), double-blind study (n =172) of a single intra-articular injection of trans-capsaicin that found a dose-dependent improvement in pain with walking in OA patients.10 Patients who received a 0.5 mg injection (n = 33) had favorable improvement (p = 0.0740) compared to placebo recipients (n = 69) at the 12-week mark, whereas those who received a 1 mg injection (n = 70) had statistically significant improvement at both 12 weeks (p < 0.0001) and 24 weeks (p = 0.0002) when compared to placebo.10 This product is currently undergoing phase 3 study (n = 332; ClinicalTrials.gov Identifier: NCT03429049).
Post-surgical pain treatment via targeted delivery of an investigational capsaicin pro-drug known as vocacapsaicin is being studied in multiple clinical trials in patients undergoing total knee arthroplasty (TKA), bunionectomy, ventral hernia repair, and complete abdominoplasty.2,11-13 This proprietary drug is delivered via a water-soluble, injectable vehicle that is rapidly converted to lipophilic capsaicin, which then disrupts the nociceptive signaling of the intended TRPV1 channel. Although the data remains to be published, promising results have been found in the TKA population after a phase 2 clinical trial comparing infiltration of the surgical site with a formulation of vocacapsaicin (36 mg arm, n = 60; or 60 mg arm, n = 61) to a standard of care arm (n = 66; spinal anesthesia, ketorolac, acetaminophen, ropivacaine regional anesthesia (joint infiltration, femoral nerve block, and infiltration between popliteal artery and capsule of the knee (IPACK) block)). Pain at rest and with ambulation both improved up to 168 hours after injection (p = 0.01 and p = 0.0072, respectively) in the 36 mg vocacapsaicin injection arm of the trial. Surprisingly, the 36 mg group’s results were consistently better than the 60 mg group’s, including greater reductions in pain and opioid consumption.14 In a recently published pilot study, vocacapsaicin 24 mg was infiltrated into the surgical site after open laparotomies and found to significantly decrease pain on coughing and pain on ambulation with no difference in safety compared to placebo.15 Ongoing trials for this drug have only recently resulted, so it is anticipated that further trials will soon be underway.
Conclusion
Since TRPV1’s role in thermosensation has been defined and drug delivery systems have become more precise, it is likely that newer modulation options will continue to be studied. There is always a possibility that the current wave of excitement regarding injectable options targeting TRPV1 for pain conditions will fizzle out like what occurred with the once hotly debated topical capsaicin. Thus far, however, the studies on injectable capsaicin are promising, and these drugs may be predecessors to a new generation of non-opioid pain control treatments.

Neel Atawala is a medical student at Mercer University School of Medicine in Savannah, GA.

Vats T. Ambai, MD, is a resident physician in the department of anesthesiology at Emory University School of Medicine, in Atlanta, GA.

Arun Kalava, MD, is an assistant professor of anesthesiology at the University of Central Florida College of Medicine in Tampa.
References
- Koivisto A-P, Belvisi MG, Gaudet R, et al. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov 2022;21:41–59. https://doi.org/10.1038/s41573-021-00268-4
- Concentric Analgesics. Study Evaluating the Safety, Efficacy and Pharmacokinetics of CA-008 (Vocacapsaicin). ClinicalTrials.gov Identifier: NCT04203537. Updated January 12, 2022. Accessed August 28, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04203537.
- Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. https://doi.org/10.1038/39807
- Iftinca M, Defaye M, Altier C. TRPV1-targeted drugs in development for human pain conditions. Drugs 2021;81:7–27. https://doi.org/10.1007/s40265-020-01429-2
- Discoveries of receptors for temperature and touch. Advanced Information Nobel Prize. https://www.nobelprize.org/prizes/medicine/2021/advanced-information. Published 2021. Accessed April 6, 2022.
- Sanz-Salvador L, Andrés-Borderia A, Ferrer-Montiel A, et al. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem2012;287:19462–71. https://doi.org/10.1074/jbc.M111.289751
- Messeguer A, Planells-Cases R, Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol 2006;4(1):1-15. https://doi.org/2174/157015906775202995
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother 2010;11:1359–71. https://doi.org/10.1517/14656566.2010.481670
- Campbell JN, Stevens R, Hanson P, et al. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules 2021;26:778. https://doi.org/10.3390/molecules26040778
- Stevens RM, Ervin J, Nezzer J, et al. Randomized, double-blind, placebo-controlled trial of intraarticular Trans-Capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol 2019;71:1524–33. https://doi.org/10.1002/art.40894
- Concentric Analgesics. Preliminary Study of CA-008 (Vocacapsaicin) in Patients Undergoing Ventral Hernia Repair. ClinicalTrials.gov Identifier: NCT04774328. Updated September 14, 2022. Accessed August 28, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04774328.
- Concentric Analgesics. Study in Subjects Undergoing Complete Abdominoplasty. ClinicalTrials.gov Identifier: NCT03789318. Updated January 13, 2022. Accessed August 28, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03789318.
- Concentric Analgesics. Study of CA-008 (Vocacapsaicin) in Bunionectomy Patients. ClinicalTrials.gov Identifier: NCT03599089. Updated August 11, 2021. Accessed August 28, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT03599089.
- Higgins O, Rule S, Cleary J. Analgesics of the future: the potential of vocacapsaicin injections for knee pain. Pract Pain Manag 2021;21(2). https://www.practicalpainmanagement.com/treatments/pharmacological/non-opioids/analgesics-future-potential-vocacapsaicin-injections-knee-pai
- Teichman S, Garza J, Kleinman M, et al. Pilot study of vocacapsaicin for treatment of pain after open laparotomy. J Am Coll Surg 2022;235(5):S23. https://doi.org/10.1097/01.XCS.0000895796.73917.3d