Surgical Considerations for Spinal Cord Stimulation Implant
Introduction
Spinal cord stimulation (SCS) is now an established therapy indicated for the treatment of chronic pain of the trunk and limbs. Specific indications may include failed back surgery syndrome, neuropathic pain (due to various etiologies including radiculopathy and peripheral neuropathy), and chronic regional pain syndrome. Critical to the success of this therapy are appropriate patient selection, diagnosis, and meticulous perioperative planning. Herein, we outline infection mitigation strategies for the implanting physician planning percutaneous spinal cord stimulator lead and battery placement.
The development and implementation of consistent, evidence-based practices regarding infection risk mitigation, wound closure, and instrumentation is needed to effectively provide high-level surgical care.
Mitigation of Infection Risk
Adherence to strict sterile operative technique during SCS trialing and implantation is mandatory. The Neurostimulation Appropriateness Consensus Committee (NACC) released guidelines that address 35 consensus points representing best practices for infection mitigation.[1] The 35 consensus points themselves, as well as the strength of recommendation, were derived from evaluation of studies from the United States Preventive Task Force hierarchies.[2] Understanding and implementing these best practices is vital to ensuring safe and successful SCS implantation.
Recently, Falowski et al[3] extracted data from the Marketscan® Commercial Claims and Encounters Database (2009-2014) and the Medicare Supplement databases (2011-2014) with the goal of estimating the risk of surgical site infection (SSI) following SCS insertion. At 12 months following SCS implantation, the risk of surgical site infection was found to be 3.11%[3], with most infections occurring within the first 90 days. Significant factors that were found to increase the risk for development of SSIs included the presence of peripheral vascular disease, history of previous infection in the 12 months prior to surgery, and younger age. Hoelzer et al conducted a retrospective review across 11 study sites (including seven academic centers) and reported a lower SSI rate of 2.45%.[4] In this review, statistically significant risk factors for infection included surgeries performed at academic centers and initial trial length exceeding five days. Significant protective factors included the application of an occlusive dressing and prescription of postoperative antibiotics (beyond 24 hours following surgery). Interestingly, neither study identified tobacco use, poorly controlled diabetes mellitus, or obesity as independent risk factors for infection. Acknowledging the limitations of a retrospective study design, the remainder of this article will attempt to describe several perioperative infection control practices that may significantly impact the development of SSIs.
In an international survey that aimed to evaluate perioperative infection control practices encompassing SCS implantation, approximately 50% of the 506 physician respondents reported extending antibiotic therapy beyond the 24-hour postoperative period for both trials and implants.[5] Multiple studies have demonstrated that there is no benefit to continuing postoperative antibiotic therapy beyond 24-48 hours following spine surgery,[6] orthopedic cases,[7],[8] maxillofacial surgery,[9] and cardiac surgery.[10] Indeed, prolongation of antibiotic therapy has been associated with an increased duration of hospital stay, longer time until normalization of body temperature, and higher serum markers of inflammation (ie, C-reactive protein) following instrumented spine surgery.[11] The World Health Organization examined the optimal duration of antibiotic prophylaxis by pooling the results of 69 randomized controlled trials that included 21,243 patients. [12] They found moderate evidence that there is no benefit to postoperative continuation of antibiotic prophylaxis beyond 24 hours, with only low-quality evidence for benefit derived from prolonged administration in select cardiac, vascular, and orthognathic surgeries. Additionally, patients who received prolonged continuation of antibiotics reported an increased frequency of clostridial enterocolitis, rash, erythema, phlebitis, hypotension, pruritus, and GI symptoms. Cost savings associated with shorter antibiotic regimens ranged from $1,644 to $3,690.[12]
Reducing SSI through mitigation of known risk factors including poorly controlled diabetes mellitus, obesity, and smoking status are critical for reducing infection risk. Diabetic patients have a 50% increased risk of SSI across surgeries in general (doubled in cardiac surgery).[13] Patients with HbA1c levels of 7.5 mg/dL demonstrated significantly higher rates of deep SSI following single-level decompression (Odds Ratio 2.9).[14] Obesity, typically defined in the literature relating to SSI as a body mass index higher than 35, is commonly identified as an independent risk factor for SSI.[15-17] In lumbar spine procedures, adipose thickness at the site of surgical incision may be a more significant factor when estimating SSI risk than obesity in and of itself.[18] History of smoking increases the risk for SSI following elective surgical procedures by 50%, and this risk is doubled if patients smoke on the day of surgery.[19] In a recent meta-analysis of 67,405 patients undergoing spine surgery, smokers had a 26% increased risk of SSI compared to non-smokers.[20]
NACC recommends the following for infection management practice (evidence level I; recommendation strength A) for both trials and implants unless otherwise indicated.
- Decolonize methicillin sensitive Staphylococcus aureus (MSSA)/ methicillin resistant Staphylococcus aureus (MRSA) carriers through application of mupirocin ointment and chlorhexidine baths.
- Use preoperative antibiotics for trials and implants using weight-based dosing and appropriate timing (within 1 hour prior to surgical incision, with the exception of vancomycin).
- When required, remove hair immediately prior to surgery with electrical clippers.
- If incise drapes are used, use iodophor-impregnated drapes.
- Use laminar flow and high-efficiency particulate air (HEPA) filters in the OR for implants.
- Limit procedure room traffic.
- Do not continue postoperative antibiotics beyond 24 hours.
NACC recommends the following for infection management practice (evidence level II; recommendation strength B) for both trials and implants unless otherwise indicated.
- Identify and treat all remote infections prior to trials and implants.
- Optimize glucose control.
- Discontinue tobacco use.
- Perform preoperative surgical scrub for a minimum of 2-5 minutes prior to surgery.
- Keep nails short, and do not wear artificial nails.
- Wear a surgical mask and cap or hood to fully cover hair.
- Use sterile gown and gloves; double glove.
- Limit operative time.
- Apply an occlusive dressing for 24-48 hours.
NACC recommends the following for infection management practice (evidence level III) for both trials and implants unless otherwise indicated.
- Do not wear arm or hand jewelry.
- Limit tissue trauma, maintain hemostasis, eradicate dead space, and avoid electrocautery at tissue surface.
- Understand maximum time criterion of 1 year for defining a deep SSI of an implantable device.
- Educate family and patient on proper incision care, symptoms of SSI, and importance of reporting symptoms.
- Wash hands before and after dressing changes, and use sterile technique.
- When SSI is suspected, prescribe an appropriate antibiotic that covers the likely causative organisms (consider local resistance patterns and culture results).
Wound Closure
In preparation for closure, adequate hemostasis and copious irrigation should occur. Appropriate wound closure should achieve the following goals: closure of dead space, support for wound during healing process, maximization of blood flow, approximation of wound edges, minimization of bleeding, mitigation of infection risk and decreased bacterial contamination, and durable cosmesis.[21] Wound edges should be everted and well approximated, and closure should provide adequate prolonged support during wound healing.
Type of Suture
Synthetic, nonabsorbable suture is used for anchoring and may include polyester (EthibondTM), polypropylene (Prolene®), or nylon (Ethilon®). Nonabsorbable sutures retain most of their tensile strength after 60 days. Although silk is classified as a nonabsorbable suture, it does degrade in tissue with a variable rate and therefore loses its tensile strength. Nylon sutures offer high tissue strength with low tissue reactivity and are inexpensive but may be more cumbersome, particularly when working in deeper fascia. Polypropylene sutures exhibit high tensile strength, low tissue reactivity, and good infection resistance but offer low elasticity. Polyester suture is braided and has significant strength and durability. A minimum of five throws are required to maintain knot security.
Absorbable sutures are used to decrease dead space and encourage subcutaneous wound approximation in deep layers prior to superficial skin closure. A common choice is polyglactin (Vicryl®) which is a braided suture. Braided suture has an increased bacterial adherence up to 10 times higher than monofilament suture.[21] Polyglactin 910 monofilament retains 40% of its integrity at 21 days, generally holding its tensile strength for up to 3 weeks, and is absorbed at 56-70 days.[22] It is easy to handle but has a high coefficient of friction when sliding through tissue. Tensile strength is generally defined as the amount of horizontal tension required to break the material and can change significantly over time. For example, the tensile strength of 4-0 Vicryl has been measured to be 13 newtons prior to immersion in tissue or solution where it might decrease by 70-80%.[22]
Superficial skin closure often involves staples or monofilament absorbable suture such as poliglecrapone (Monocryl®) with or without Steri StripsTM or adhesive for reinforcement. Monocryl retains 60-70% of its original strength at 7 days post-implant and 30-40% strength at 14 days post-implant.[22] It will dissolve no later than 4 months after implant (91-119 days). It offers high tensile strength with low tissue reactivity and, although technically absorbable, may be removed if preferred by the patient. The choice of suture or staples for superficial skin closure is left to the surgeon. Studies evaluating sutures versus staples for skin closure and their impact on SSI have demonstrated conflicting results. Staples have been associated with an increased risk of infection in some surgical settings compared to subcuticular closures.[23] In a meta-analysis of non-orthopedic surgeries involving more than 2,000 patients, the use of staples was associated with faster closure times (>5 minutes difference between groups), but were also associated with higher rates of infection and, in several studies, higher levels of pain.[24] A meta-analysis of orthopedic surgeries found higher rates of infection associated with staple closure compared to suture (relative risk 3.83), although the included studies were of poor methodological quality.[25] A meta-analysis evaluating skin closure techniques after total knee arthroplasty demonstrated that skin sutures had a higher likelihood of superficial and deep infections, abscess formation, and wound dehiscence.[26] Other considerations include efficiency (favoring staples), cost (neutral), comfort (favoring suture), and cosmetic appearance (favoring suture). Thin skin or “neurogenic” skin, commonly found in patients with spinal cord injury, may lead a surgeon to favor closure with thin, absorbable suture to preserve skin integrity. Staple closure may be associated with more superficial drainage that may be distressing to some patients. In conclusion, neither approach has been shown to be superior in terms of scar outcome or infection rates.[27],[28]
It is important for the surgeon to identify their preference with respect to suture needle tip, but utilization may largely be driven by equipment available in their hospital or surgical center. Needle types include taper point, blunt taper point, tapercut, cutting, reverse cutting, and micro-point spatula. Type of point is left to the surgeon’s preference and discretion, but cutting type points pass through tissue with less trauma. Needle shape may be straight (not generally recommended) or curved (1/4, 3/8, 1/2, or 5/8 circle).
Understanding Surgical Glue
Skin adhesive (most commonly cyanoacrylate tissue adhesive) provides structural integrity to suture used in superficial closure and may provide a microbial barrier for up to 72 hours after closure. However, there is a low risk of skin reaction/irritation with skin adhesive that may be mistaken for an infection or allergic reaction. If cyanoacrylate tissue adhesive is used, the surgeon must first cleanse the skin after closure and take care to ensure that edges are approximated such that the adhesive does not seep into the wound. Adhesive application results in an instant seal over wound edges, and glues edges together until it peels off over the course of 7-14 days.
Surgical strips may stay in place longer if applied over skin that is first cleaned with preapplication liquid adhesive (eg, Mastisol®). Surgical strips may be used to help bolster atrophic skin as well. Sensitivity may also occur with Mastisol, and history of skin sensitivity should be elicited from patients in whom SCS implant is being considered.
Drapes and Surgical Dressings
Iodophor-impregnated drapes may reduce the risk of bacterial colonization but have not been shown to definitively decrease the risk of SSI.[29] NACC guidelines do not strongly support their use, but they may be considered in patients at elevated risk for SSI. Some surgeons elect to use iodophor-impregnated drapes for implants only.
In contrast, non-iodophor-impregnated drapes increase the risk for infection and are not recommended. Similarly, there is insufficient evidence to establish that silver impregnated surgical dressings reduce the risk of SSI.
Surgical Instruments
The types of surgical instruments employed are at the discretion of the surgeon. Figure 1 provides an example of tray setup.
Specific attention should be paid to surgical instrument preference for identifying and separating tissue planes. One approach that may reduce the need for cautery and improve wound healing includes employing a Weitlaner retractor to identify the natural tissue plane with the midline incision for implants. Blunt dissection can then be used as needed to facilitate anchor deployment and suturing, with instrumented dissection only employed as necessary.
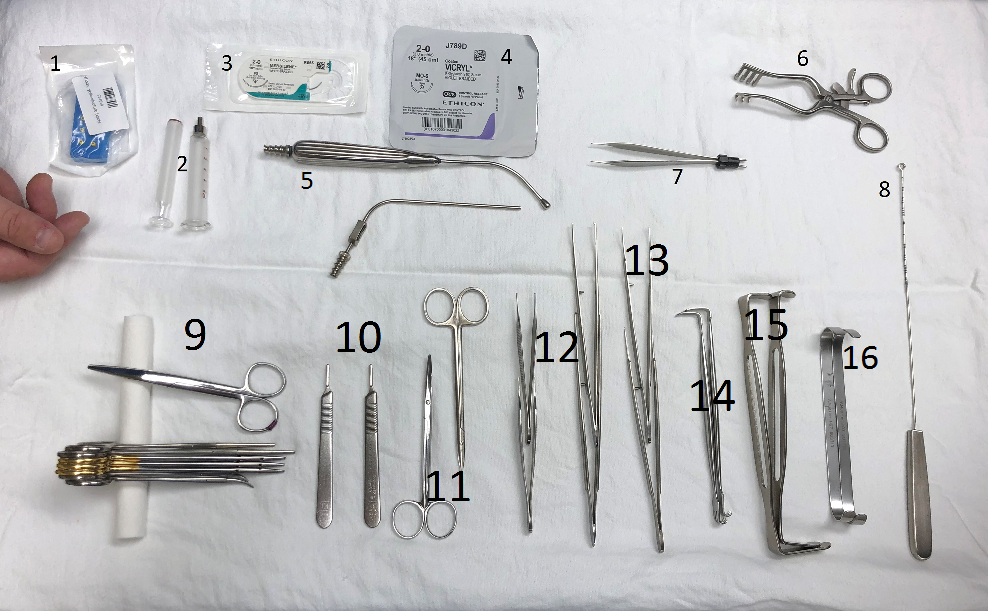
Figure 1: Tray setup. (Please note this is an example of surgical supplies commonly used for spinal cord stimulator implants but can vary based on different practice settings.)
1) needle holder; 2) loss of resistance syringe; 3) anchor suture; 4) deep closure suture; 5) irrigation; 6) Weitlaner retractor; 7) bipolar diathermy; 8) radiopaque marker; 9) Mayo (suture) scissors; 10) scalpel handles; 11) tenotomy scissors; 12) tissue forceps; 13) Gerald tissue forceps; 14) Senn retractors; 15) Army-Navy retractors; 16) Farabeuf retractors.
Discussion
The science and practice of neuromodulation for the treatment of chronic neuropathic pain continues to evolve as appropriate indications and patient candidacy are better understood. Although practiced for more than 50 years, the advances made in only the past 10-15 years have greatly expanded the accessibility of this therapy to appropriate patient populations.
As we seek to offer effective treatments to patients with the goal of improving pain and function, we must bear in mind the incredible clinical, humanistic, and economic costs to patients and the healthcare system. The cost of SCS-related SSI is high, with incremental annual healthcare expenditures for patients with an SCS-related SSI estimated to be nearly $60,000 for initial implants and nearly $65,000 for replacements.[30] The majority (73-77%) of infected SCS systems ultimately require explant, and most patients never undergo reimplantation.[31] In the international survey discussed earlier, compliance with guidelines from the Centers for Disease Control and Prevention, National Institute for Health and Care Excellence, and the Surgical Care Improvement Project was low, with only 4 of the 15 questions related to infection control practices reporting greater than 80% compliance.[5] In fact, only 8% of respondents were even aware that a deep SSI is defined as an infection occurring up to 365 days following an implant. The survey highlights that significant opportunity for improvement in the knowledge of best infection control practices and compliance with evidence-based guidelines.
The preceding discussion is designed to address surgical considerations when proceeding to trial and/or implantation. The development and implementation of consistent, evidence-based practices regarding infection risk mitigation, wound closure, and instrumentation is needed to effectively provide high-level surgical care. The NACC guidelines provide a helpful framework for considering best evidence-based practice for reducing infection risk.
References
- Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) recommendations for infection prevention and management. Neuromodulation. 2017;20(1):31-50. https://doi.org/10.1111/ner.12565
- Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21-35. https://doi.org/10.1016/j.amepre.2020.01.001
- Falowski SM, Provenzano DA, Xia Y, Doth AH. Spinal cord stimulation infection rate and risk factors: results from a United States payer database. Neuromodulation. 2019;22(2):179-89. https://doi.org/10.1111/ner.12843
- Hoelzer BC, Bendel MA, Deer TR, et al. Spinal cord stimulator implant infection rates and risk factors: a multicenter retrospective study. Neuromodulation. 2017;20(6):558-62. https://doi.org/10.1111/ner.12609
- Provenzano DA, Deer T, Phelps AL, et al. An international survey to understand infection control practices for spinal cord stimulation. Neuromodulation. 2016;19(1):71-84. https://doi.org/10.1111/ner.12356
- Kakimaru H, Kono M, Matsusaki M, Iwata A, Uchio Y. Postoperative antimicrobial prophylaxis following spinal decompression surgery: is it necessary? J Orthop Sci. 2010;15(3):305-9. https://doi.org/10.1007/s00776-010-1464-2
- Vargas-Mena R, Arredondo-Gomez E, Pavia-Carrillo EF. Effect of a short antimicrobial prophylaxis regimen on the prevalence of postoperative infection in elective orthopedics and traumatology surgery. Acta Ortop Mex. 2012;26(6):369-74.
- Mathur P, Trikha V, Farooque K, et al. Implementation of a short course of prophylactic antibiotic treatment for prevention of postoperative infections in clean orthopaedic surgeries. Indian J Med Res. 2013;137(1):111-6.
- Bartella AK, Lemmen S, Burnic A, et al. Influence of a strictly perioperative antibiotic prophylaxis vs a prolonged postoperative prophylaxis on surgical site infections in maxillofacial surgery. Infection. 2018;46(2):225-30. https://doi.org/10.1007/s15010-017-1110-4
- Bucknell SJ, Mohajeri M, Low J, McDonald M, Hill DG. Single-versus multiple-dose antibiotics prophylaxis for cardiac surgery. Aust N Z J Surg. 2000;70(6):409-11. https://doi.org/10.1046/j.1440-1622.2000.01837.x
- Ohtori S, Inoue G, Koshi T, et al. Long-term intravenous administration of antibiotics for lumbar spinal surgery prolongs the duration of hospital stay and time to normalize body temperature after surgery. Spine (Phila Pa 1976). 2008;33(26):2935-7. https://doi.org/10.1097/BRS.0b013e3181895939
- World Health Organization. Global guidelines for the prevention of surgical site infection. https://apps.who.int/iris/bitstream/handle/10665/250680/9789241549882-eng.pdf?sequence=8. Published 2018. Accessed July 10, 2020.
- Martin ET, Kaye KS, Knott C, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):88-99. https://doi.org/10.1017/ice.2015.249
- Cancienne JM, Werner BC, Chen DQ, Hassanzadeh H, Shimer AL. Perioperative hemoglobin A1c as a predictor of deep infection following single-level lumbar decompression in patients with diabetes. Spine J. 2017;17(8):1100-5. https://doi.org/10.1016/j.spinee.2017.03.017
- Fei Q, Li J, Lin J, et al. Risk factors for surgical site infection after spinal surgery: a meta-analysis. World Neurosurg. 2016;95:507-15. https://doi.org/10.1016/j.wneu.2015.05.059
- Castle-Kirszbaum MD, Tee JW, Chan P, Hunn MK. Obesity in neurosurgery: a narrative review of the literature. World Neurosurg. 2017;106:790-805. https://doi.org/10.1016/j.wneu.2017.06.049
- Cao J, Kong L, Meng F, Zhang Y, Shen Y. Impact of obesity on lumbar spinal surgery outcomes. J Clin Neuro. 2016;28:1-6. https://doi.org/10.1016/j.jocn.2015.10.034
- Lee JJ, Odeh KI, Holcombe SA, et al. Fat thickness as a risk factor for infection in lumbar spine surgery. Orthopedics. 2016;39(6):e1124-e8. https://doi.org/10.3928/01477447-20160819-05
- Nolan MB, Martin DP, Thompson R, Schroeder DR, Hanson AC, Warner DO. Association between smoking status, preoperative exhaled carbon monoxide levels, and postoperative surgical site infection in patients undergoing elective surgery. JAMA Surg. 2017;152(5):476-83. https://doi.org/10.1001/jamasurg.2016.5704
- Kong L, Liu Z, Meng F, Shen Y. Smoking and risk of surgical site infection after spinal surgery: a systematic review and meta-analysis. Surg Infect (Larchmt). 2017;18(2):206-14. https://doi.org/10.1089/sur.2016.209
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40 Suppl 9:S3-S15. https://doi.org/10.1097/01.DSS.0000452738.23278.2d
- Khiste SV, Ranganath V, Nichani AS. Evaluation of tensile strength of surgical synthetic absorbable suture materials: an in vitro study. J Periodontal Implant Sci. 2013;43(3):130-5. https://doi.org/10.5051/jpis.2013.43.3.130
- Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, Macones GA, Odibo AO. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682-90. https://doi.org/10.1097/AOG.0b013e31820ad61e
- Iavazzo C, Gkegkes ID, Vouloumanou EK, Mamais I, Peppas G, Falagas ME. Sutures versus staples for the management of surgical wounds: a meta-analysis of randomized controlled trials. Am Surg. 2011;77(9):1206-21.
- Smith TO, Sexton D, Mann C, Donell S. Sutures versus staples for skin closure in orthopaedic surgery: meta-analysis. BMJ. 2010;340:c1199. https://doi.org/10.1136/bmj.c1199
- Kim KY, Anoushiravani AA, Long WJ, Vigdorchik JM, Fernandez-Madrid I, Schwarzkopf R. A meta-analysis and systematic review evaluating skin closure after total knee arthroplasty-what is the best method? J Arthroplasty. 2017;32(9):2920-7. https://doi.org/10.1016/j.arth.2017.04.004
- Krishnan R, MacNeil SD, Malvankar-Mehta MS. Comparing sutures versus staples for skin closure after orthopaedic surgery: systematic review and meta-analysis. BMJ Open. 2016;6(1):e009257. https://doi.org/10.1136/bmjopen-2015-009257
- Glennie RA, Korczak A, Naudie DD, Bryant DM, Howard JL. MONOCRYL and DERMABOND vs staples in total hip arthroplasty performed through a lateral skin incision: a randomized controlled trial using a patient-centered assessment tool. J Arthroplasty. 2017;32(8):2431-5. https://doi.org/10.1016/j.arth.2017.02.042
- Rezapoor M, Tan TL, Maltenfort MG, Parvizi J. Incise draping reduces the rate of contamination of the surgical site during hip surgery: a prospective, randomized trial. J Arthroplasty. 2018;33(6):1891-5. https://doi.org/10.1016/j.arth.2018.01.013
- Provenzano DA, Falowski SM, Xia Y, Doth AH. Spinal cord stimulation infection rate and incremental annual expenditures: results from a United States payer database. Neuromodulation. 2019;22(3):302-10. https://doi.org/10.1111/ner.12939
- Bendel MA, O'Brien T, Hoelzer BC, et al. Spinal cord stimulator related infections: findings from a multicenter retrospective analysis of 2737 implants. Neuromodulation. 2017;20(6):553-7. https://doi.org/10.1111/ner.12636
Leave a commentOrder by
Newest on top Oldest on top