COVID-19 DEA Guidance
The COVID-19 pandemic and public health emergency has created significant challenges relevant to healthcare access for chronic pain patients. Telemedicine visits via telephone or video encounters have replaced face-to-face interactions. In addition to the difficulties and risks associated with the inability to perform both physical examinations and interventional procedures, the presence of stringent federal Drug Enforcement Administration (DEA) guidelines for opioid prescribing, originally created to increase protection for both health care practitioners and the public, have now become occasional barriers to medication access during the COVID-19 pandemic.
When the public health emergency eventually ends and we move into a “new normal,” pain management specialists should consider the benefits and consequences of the significant flexibilities . . . provided by federal and state governments and their impact on patient care . . .
In order to facilitate medication delivery and attempt to minimize exacerbations in pain, pharmacologic withdrawal, and a variety of untoward consequences that might result in an unnecessary healthcare burden and increase in emergency room visits, the DEA has relaxed several regulations to accommodate both prescribers and patients during the current pandemic. These changes were enacted March 31, 2020, and all will extend through the end date of the public health emergency. Listed below are the most relevant points that might impact prescribers of Schedule II controlled substances (ie, most opioid agonist medications) and prescribers of buprenorphine for opioid use disorder (OUD). The DEA disclaims in several locations that these guidance documents are not binding and do not have the force or effect of law.
For providers that prescribe Schedule II controlled substances:
- Previously, all patients prescribed opioid therapy required an in-person follow-up every three months to meet DEA criteria. Currently, in patients who have already had an in-person evaluation, any method of follow-up (in-person, two-way telemedicine, telephone, e-mail, etc.) is considered an acceptable form of patient evaluation to provide three additional months of medication.
- Initiation of all controlled substances (Schedules II-V) for new patients requires either an in-person or two-way, audiovisual, real-time telemedicine visit.
- Guidelines regarding prescribing across state lines have temporarily been relaxed (but not fully removed).
- Refills for controlled substances that are Schedules III-V can continue to be called into pharmacies as usual.
- While electronic or paper prescriptions are still preferred for Schedule II medications, prescribers can now temporarily call in Schedule II prescriptions in extreme circumstances, provided that the following four conditions are all met:
- Immediate administration is necessary for proper patient treatment.
- No other appropriate alternative (non-controlled-substance) treatment is available.
- It is not reasonably possible for the prescriber to provide a written or electronic prescription.
- The prescriber provides an electronic, scanned, or copy of a written prescription to the pharmacy within 15 days.
For DATA 2000 X-waivered prescribers who manage buprenorphine therapy for opioid use disorder:
- Treatment can be initiated by any method of evaluation (in-person, telemedicine via telephone for video).
- Patients on established buprenorphine therapy can continue to have it prescribed after any method of follow-up visit (in-person, real-time telemedicine via telephone or video, e-mail, etc).
- As buprenorphine is a Schedule III medication, refills can be given as usual and it can be called in to the pharmacy in the event that electronic or written prescriptions cannot be provided.
Figure 1 presents a decision tree on how to prescribe controlled substances to patients during the public health emergency. This and further details and guidance related to managing opioid and buprenorphine therapy during the COVID-19 pandemic, can be found at: https://www.deadiversion.usdoj.gov/coronavirus.html.
In addition, the American Medical Association (AMA) released policy recommendations to help guide state policymakers as they consider options to ensure access to medication for patients with both OUD and chronic pain during the public health emergency. The AMA’s recommendations include:
- Removing restrictions on Medicaid preferred drug lists to help avoid medication shortages. This includes ensuring coverage for methadone for patients receiving care in an opioid treatment program.
- Waiving testing requirements and in-person counseling requirements for refills for patients with chronic pain and allow for telephonic counseling to fulfill state prescribing and treatment requirements
- Assisting harm-reduction organizations to help ensure adequate supplies of naloxone to continue community-based naloxone distribution efforts
- Ensuring continuity of syringe services programs, including provision of personal protection equipment (PPE)
- Expanding PPE priority to include harm-reduction organizations and other community-based organizations that provide services to people who inject drugs to help protect against the spread of infectious disease.
The AMA also is urging states to adopt policies, such as those included in a new Minnesota law[[1]], to allow Schedule II-V controlled substances to be dispensed for more than 30 days and remove existing refill limitations, during the public health emergency. Read more on the AMA’s policy recommendations here: https://www.ama-assn.org/delivering-care/public-health/covid-19-policy-recommendations-oud-pain-harm-reduction.
Moving Forward
When the public health emergency eventually ends and we move into a “new normal,” pain management specialists should consider the benefits and consequences of the significant flexibilities, including key policy revisions and relaxed enforcement, provided by federal and state governments and their impact on patient care, although many of these will likely come to an end as the public health emergency concludes. Pain management specialists and their patients may also come to realize the value of delivering and receiving health care in a virtual environment and wish to see these flexibilities made permanent.
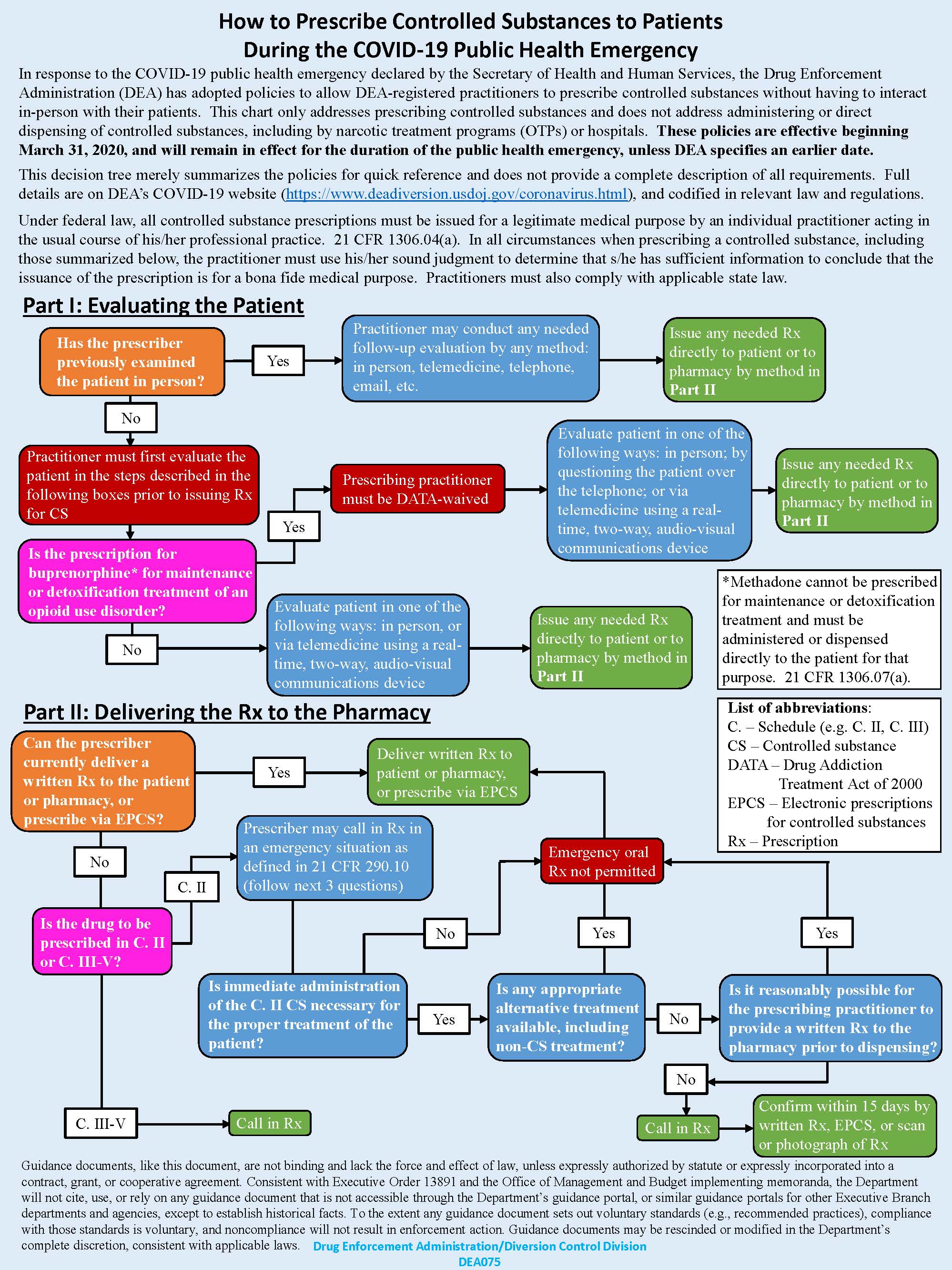
Figure 1. Controlled substances decision tree for provider
From Drug Enforcement Administration/Diversion Control Division. Available at: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Accessed June 23, 2020.
Reference
- Minnesota Legislature. Minnesota session laws - 2020, regular session. Available at: https://www.revisor.mn.gov/laws/2020/0/Session+Law/Chapter/71/ Accessed June 23, 2020.
Leave a commentOrder by
Newest on top Oldest on top