COVID-19 Risk Mitigation for Return to Practice for Chronic Pain Physicians: Risk Stratification Guidelines and Practice Management Tools
The COVID-19 pandemic has upended life across the globe and created unprecedented challenges to society in general and healthcare systems around the globe. Significant apprehension exists as chronic pain physicians contemplate how best to reopen their doors. In the United States, a prerequisite for hospitals to return to providing interventional pain procedures may include robust testing for healthcare professionals with emerging antibody testing.[1] Because conditions are highly variable across the United States with COVID-19 outbreaks ranging from mild to severe, state and local authorities adjust recommendations based on their current situation. To better serve the chronic pain community during this challenging pandemic, the American Society of Interventional Pain Physicians (ASIPP) established the COVID ASIPP Risk Mitigation and Stratification (COVID-ARMS) Return to Practice Task Force, chaired by Dr. Shalini Shah. The mission of the task force was to develop strategies aimed at mitigating risks for clinicians, providers, and patients.
As interventional pain practices reopen, there is an ethical imperative to inform patients of the risks and benefits of pain treatment in the era of COVID-19.
The federal “Opening Up America Again” program in the United States is a three-phase, data-driven approach guided by public health experts that provides guidance for healthcare systems.[1] A core responsibility at the state level for preparedness of healthcare systems requires the ability to handle the supply and demand requirements for personal protective equipment (PPE) and critical medical supplies throughout hospitals systems.[1] Because of the discrepancy in the supply and demand needs during the COVID-19 outbreak, non-urgent or emergent care had temporarily been postponed for many patients, including chronic pain patients. Chronic pain afflicts at least 50 million Americans, of whom about 20 million have “high impact” chronic pain with functional deficits.[2] Overlooking routine care for chronic pain patients can have negative impacts on health, well-being, emotional stability, function, and quality of life.[3] Patients under the care of interventional pain specialists require interdisciplinary care and ongoing procedures to control their disease pathology. The ASIPP COVID-ARMS Return to Practice Task Force was established to provide a strategy for the reopening of interventional pain practices by applying risk mitigation and stratification algorithms. Risk mitigation incorporates specific steps to reduce harm to patients, healthcare professionals, and the healthcare system. This requires the identification and assessment of risk factors, review of emerging literature, ongoing data analysis, training and education, and creating organizational responses to the pandemic. Risk stratification relies on triaging capabilities and identifying risks that increase morbidity or mortality. The COVID-ARMS Task Force sought to offer guidance that was not overly prescriptive, in that the best care is provided when careful clinical judgment is exercised on a case-by-case basis. A COVID-ARMS scoring system was created to objectively risk-stratify patients. Clinicians base their medical decisions by assessing individual risks versus benefits. With the SARS-nCoV-2 virus, our decisions have become dynamic and situational, as more evidence and elucidation of the virus emerges. Recommendations that are appropriate in one setting and location may be inappropriate in another. It is important for interventional pain specialists to take a localized approach to resuming their practice.
Specific Comorbidities and Methodology of Risk Stratification Guidelines
The COVID-NET survey conducted by the Centers for Disease Control and Prevention (CDC) on hospitalized COVID-19 patients reported a hospitalization rate of 4.6 per 100,000 population and observed that 90% of hospitalized patients with COVID-19 had one or more underlying medical conditions, of which hypertension, obesity, chronic lung disease, type 2 diabetes, and cardiovascular disease were most common.[4] Age was found to be a predictive factor for COVID-19 hospitalizations, with older age associated with higher rates of hospitalization.[4] Patient comorbidities varied with different age groups (Table 1). A single-center study from Wuhan China reported 32% of the patients diagnosed with COVID-19 in early January had comorbid conditions, of which the most frequently reported were diabetes (20%), hypertension (15%), and cardiovascular disease (15%).[5] The authors also reported that 49% of patients were between the ages of 25-49 and 34% were between the ages of 50-64.[5] In a study from Korea, 90.7% of all fatalities in COVID-19 involved a patient with at least one comorbidity, the most frequently reported of which were hypertension, cardiovascular conditions, diabetes, dementia, and stroke.[6]
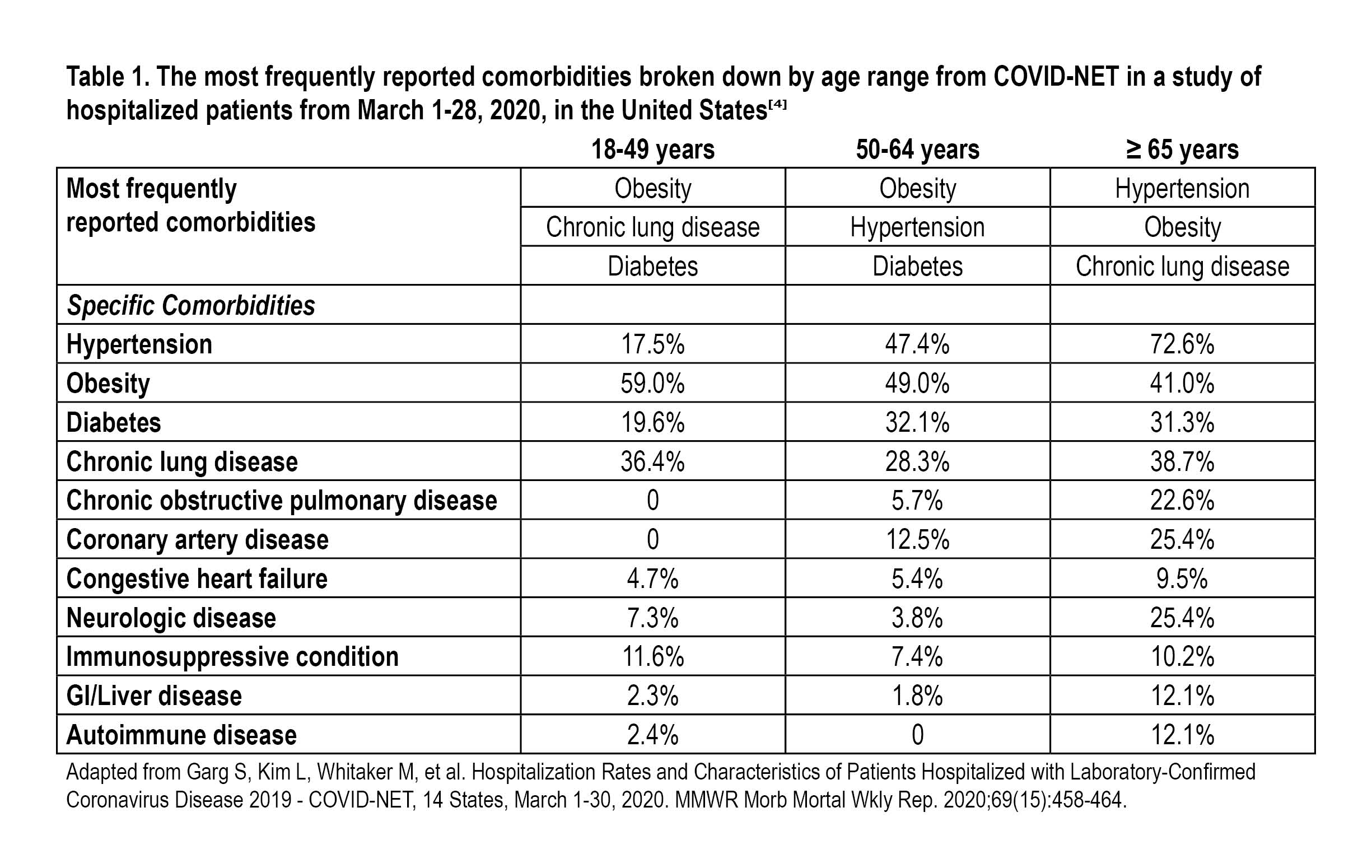
Patients with specific underlying conditions may have an increased risk for more severe or negative outcomes with COVID-19 than people who do not have these underlying conditions.[7] The China Centers for Disease Control and Prevention published case fatality rates for the following comorbidities: hypertension 6.0%, diabetes 7.3%, cardiovascular disease 10.5%, chronic respiratory disease 6.3%, and cancer 5.6%.[8]
In a study of 52 critically ill COVID-19 patients (mean age 59.7 years), 20 survived and 32 died.[9] When comparing survivors to non-survivors, survivors had lower rates of comorbidities (20% vs. 53%, respectively); the most commonly occurring comorbidities reported for survivors versus non-survivors were cardiovascular disease (20% vs. 53%) and cerebrovascular disease (0 vs. 22%).[10] In addition to comorbid disease, risk factors for COVID-19 also include advanced age, nursing home or congregated residences, and immune suppression. While COVID-19 infects both sexes equally, men have a higher case fatality rate than women (3.6% vs. 1.6%).[11]
In a study of 1,591 COVID-19 patients from the Lombardy region in Italy, it was found that patients ≥ 64 years had a higher mortality rate than those ≤ 63 years (36% vs. 15%, respectively, 95% confidence interval [CI], 17% to 26%, p<0.001).[12] In a study of 5,700 COVID-19 patients at 12 hospitals in the area in and around New York City from March 1-April 4, 2020, outcomes were assessed in 2,634 patients who were discharged or died. Of these patients, 14.2% were treated in an ICU (median age 68 years, interquartile range 56 to 78 years) and 21% died; the median age of the general population in New York City is 35.8 years.[13] In a retrospective study from a single center in Wuhan, China, 49.1% of a total of 548 admitted for COVID-19 had severe disease with older age a risk factor for severe COVID; mortality for COVID-19 was 1.1% in non-severe and 32.5% in severe cases over a mean 32 days of follow-up.[14] New York City Health Department daily death summaries dated April 14, 2020, showed that patients with increasing age with or without underlying conditions had a higher risk of mortality.[15]
The task force created risk stratification guidelines (Table 2) and flow chart (Figure 1) to assist clinicians in restarting their interventional pain practices based on the data published from Wuhan, autopsies from expired COVID patients, the COVID-NET survey, and from surveillance studies.
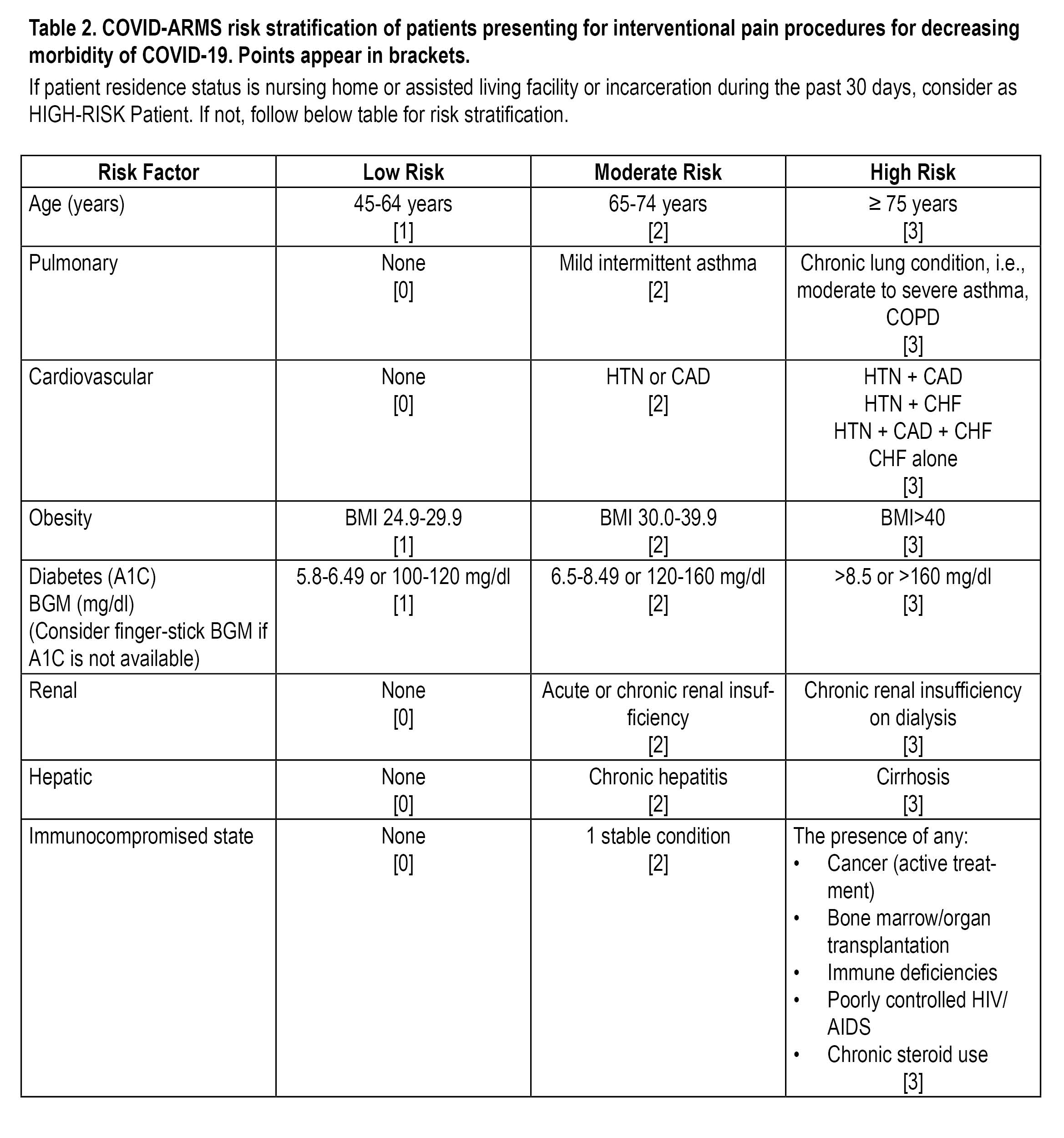
Patients who score <7 may be considered low risk, those scoring 8-14 are moderate risk, and high-risk patients are those who score ≥ 15.
BGM blood glucose meter; BMI body mass index; CAD coronary artery disease; CHF congestive heart failure; COPD chronic obstructive pulmonary disease; HTN hypertension
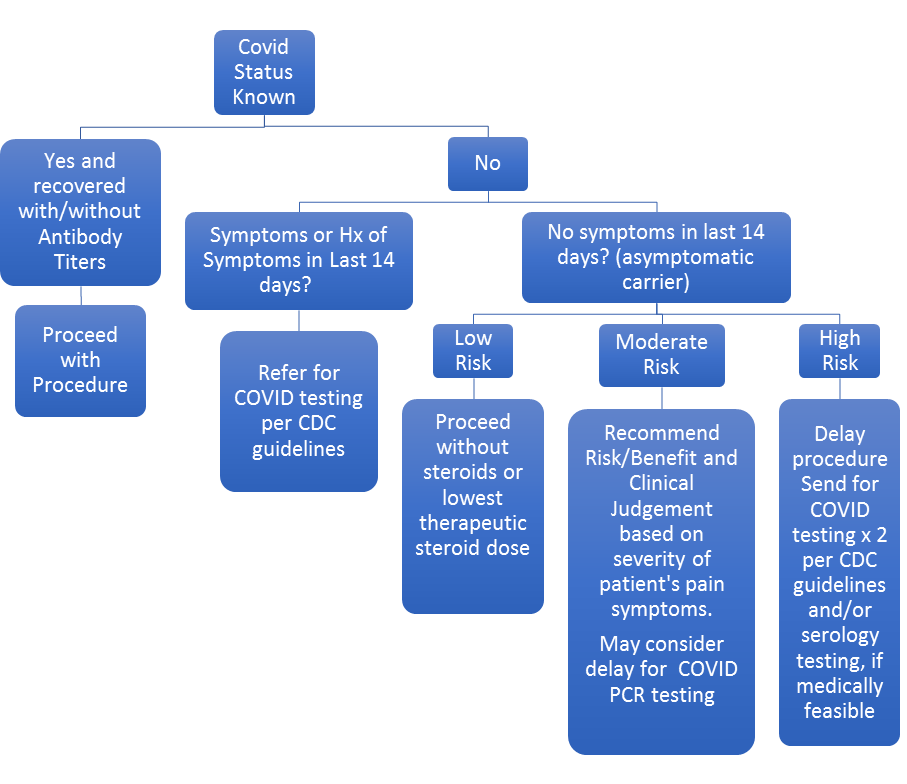
Figure 1. ASIPP COVID-19 morbidity risk mitigation flow chart for interventional pain management during phase 1 reopening
Disclaimer: These documents represent a toolkit of information to help you assess risks of elective pain management procedures. They should not be construed as standard of care. Ultimately, physician discretion prevails above all and there could be scenarios where patients who are high risk can benefit and safely receive a procedure. In high-risk patients, we suggest that physicians try to mitigate risks, inform patients of risks, and proceed with caution if the benefits outweigh the risks. While testing all patients would be ideal, we realize it also may be impractical; therefore, making sure you make best efforts to prevent disease spread and efforts to mitigate risks of spread is important. Use in conjunction with state regulation.
Steroids and Steroid-Related Immune Suppression for Pain Procedures
Corticosteroid-related immunosuppression was first demonstrated in an animal model of allograft rejection in the 1950s.[16] Although corticosteroid-related immunosuppression has not been a significant consideration in interventional pain medicine prior to the COVID-19 era, concerns about the effects of injectable steroids on the immune system are being actively discussed in the interventional pain medicine community.[17] Corticosteroids exert a variety of effects that may be either beneficial or deleterious to optimal outcomes in interventional pain medicine. There are many dose-related adverse effects of corticosteroids, ranging from osteoporosis and related consequences, such as vertebral compression fractures, avascular necrosis, delayed wound healing, ecchymosis, adipose tissue redistribution leading to a “buffalo hump,” painful myopathy, diabetes, and neuropsychiatric changes, among many others.[18,19]
The immunological effects relevant to COVID include elevated glucose levels that predispose an individual to an infection, migratory inhibition of leukocytes, reduced production of inflammatory mediators such as interleukins and macrophage migration inhibition factor (MIF), inducing sequestration of CD4+ T-lymphocytes in the reticuloendothelial system (RES), and inhibitory effects on proliferation and function of lymphocytes via inhibition of lymphokines and cytokines.[20]
Given the equivocal evidence for steroids in many interventional techniques together with the current lack of effective therapeutic defenses against COVID-19, clinicians should carefully consider their use at this time, particularly the use of high doses in patients with increased risk. In cases with an inflammatory component leading to an exacerbation in pain, a lower than normal dose of steroids should be considered.
While steroid use has been shown to increase the risk of influenza,[21] there are no studies looking at the effect of steroids and COVID-19. It had been speculated that steroids might have a beneficial effect on the course of COVID-19 given results from a retrospective study of patients infected in Guangzhou, China, with the older SARS virus. The use of corticosteroids was associated with lower mortality and shorter hospital lengths of stay among critical patients.[22] In this study, critical patients were treated with a mean daily dose of corticosteroids of 133.5 ± 102.3 mg and noncritical patients received a mean daily dose of 105.3 ± 86.1 mg. While SARS-CoV-1 and SARS-nCoV-2 are related viruses, it is unclear if results with an earlier coronavirus can be generalized to COVID-19 patients. A case study in the literature reported on a familial cluster of COVID-19 patients in China taking long-term steroid therapy; COVID-19 was diagnosed in three family members but had atypically long incubation periods, exceeding the usual 14-day quarantine recommendation.[23] Median incubation period for COVID-19 was 5.1 days (95% confidence interval, 4.5 to 5.8 days) and <3% develop symptoms after 11.5 days; the recommended quarantine period is 14 days.[23] The use of steroids was associated with atypical disease presentation but not worse outcome.
Practice Management Considerations: How to Develop Office and Ambulatory Surgical Center Protocols and Informed Consent Disclosures
As healthcare providers, it is important that we recognize that our patients may be confused, distressed, or angry, particularly if they need elective surgery or procedures. As interventional pain practices reopen, there is an ethical imperative to inform patients of the risks and benefits of pain treatment in the era of COVID-19. A specific informed consent for COVID-19 (Figure 2) may be helpful as it sets forth the risks and benefits of treatment in the context of the disease in layman’s terms. It can improve documentation, enhance education, and outline key talking points that should be discussed and understood by the patient. It also serves as a source of information for the patient to share with family and caregivers.
Please review the following document (Figure 2) for relevance to your practice and modify it as you see fit. This document is a modification of the Soin-Buenaventura Consent document.
 Figure 2: Sample Risk Disclosure/ Informed Consent Form
Figure 2: Sample Risk Disclosure/ Informed Consent Form
(Click on the image to download.)
It is important for clinicians to be aware of local and state regulations. The local or state department of health and/or board of medical licensure offers guidance and direction for appropriate protocols for the return to practice. Clinicians should create protocols that address clinical operations for their office in the time of COVID-19 and the screening process for patients. Patients may be required to have their temperature taken and be asked questions about recent exposure to an infected person, recent travels, and current symptoms. Clinical staff are encouraged to sanitize hands frequently and maintain social distancing. Should a confirmed case of exposure be identified, contact tracing is encouraged to prevent further spread of the disease. If the clinician or clinical team comes in close contact with an infected person, self-isolation may be considered in order to prevent transmission of the virus; the self-quarantine is recommended for 14 days or until a negative COVID-19 test result can be confirmed.[24,25] It is important to align with your state and institutional policies when creating these protocols.
The COVID-19 pandemic has significantly impacted the healthcare system. Unlike other nonessential businesses, healthcare for managing pain cannot simply be shut down for weeks. Many chronic pain patients depend on interdisciplinary care and interventional procedures to diagnose their condition and ease their suffering, along with improve their function and quality of life. During this time of pandemic and social uncertainty when otherwise healthy people feel anxiety, a sense of loss, and depression, chronic pain patients are particularly vulnerable to psychological distress. Interventional pain specialists must lead the way as America reopens and clinical services resume. Our patients depend on us for their quality of life. During this most challenging time, we must be supportive, encouraging, and truthful, but also cautious, and realistic. Communication and science are our best tools in the armamentarium against COVID-19. By stratifying patients for risk, testing when indicated, delaying procedures when appropriate, and following local guidance, quality care can be effectively and safely resumed.
Acknowledgment: This article was edited by Dalia H. Elmofty, MD, FASA, associate professor, University of Chicago.
References
- Office of the White House. Guidelines: Opening up America again. Available at: https://www.whitehouse.gov/openingamerica/. Accessed April 28, 2020.
- Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-6.
- Cortiula F, Pettke A, Bartoletti M, Puglisi F, Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31(5):553-5.
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-64.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Korean Society of Infectious Diseases, Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132. Doi: 10.3346/jkms.2020.35.e132
- CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382-6.
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China 2020. CDC Weekly. 2020;2(8):113-22.
- Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020 Jul;111:102487. Epub 2020 May 15. Doi: 10.1016/j.jaut.2020.102487.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. 2020 May;8(5):475-81. Epub 2020 Feb 24.doi: 10.1016/S2213-2600(20)30079-5.
- Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1286. Online ahead of print.
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with sars-cov-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 Apr 6;323(16):1574-81. doi: 10.1001/jama.2020.5394. Online ahead of print.
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 Apr 22;323(20):2052-9. doi: 10.1001/jama.2020.6775. Online ahead of print.
- Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 Apr 12:S0091-6749(20)30495-4. doi: 10.1016/j.jaci.2020.04.006. Online ahead of print.
- New York City Health. Coronavirus disease 2019 (COVID-19) daily data summary. New York City Health. Available at: https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-19-daily-data-summary-deaths-04152020-1.pdf. Accessed May 10, 2020.
- Burns CM. The history of cortisone discovery and development. Rheum Dis Clin North Am. 2016;42(1):1-14, vii.
- Hackett BA. Providing steroid/corticosteroid injections safely in the Covid-19 environment. J Radiol Nurs. 2020 May 3. doi: 10.1016/j.jradnu.2020.04.008. Online ahead of print.
- Berthelot JM, Le Goff B, Maugars Y. Side effects of corticosteroid injections: what's new? Joint Bone Spine. 2013;80(4):363-7.
- Frenkel B, White W, Tuckermann J. Glucocorticoid-Induced Osteoporosis. Advances in experimental medicine and biology. 2015;872:179-215.
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2-13.
- Sytsma TT, Greenlund LK, Greenlund LS. Joint Corticosteroid Injection Associated With Increased Influenza Risk. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):194-8.
- Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441-52.
- Han Y, Jiang M, Xia D, et al. COVID-19 in a patient with long-term use of glucocorticoids: A study of a familial cluster. Clin Immunol. 2020;214:108413.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med 2020;172(9):577-82.
- Characteristics of Health Care Personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477-81.
Leave a commentOrder by
Newest on top Oldest on top