Exosomes: The Good, The Bad, and The Ugly
Cite as: Hanyu-Deutmeyer A, Buchheit T. Exosomes: the good, the bad, and the ugly. ASRA Pain Medicine News 2023;48. https://doi.org/10.52211/asra050123.009.
Introduction
The last decade has seen an explosion of interest in regenerative medicine. From prolotherapy to platelet-rich plasma to stem cells, the quest to reverse disease has captured the attention of researchers worldwide. Interest in exosomes as an additional therapeutic option for osteoarthritis (OA) and neuropathy has also grown tremendously. Although originally thought to function as a cellular housekeeping tool, removing unwanted cellular waste,1 exosomes are now known to be involved in numerous biological processes and cell functions. These small vesicles have a long circulating half-life, can easily penetrate cell membranes, cross the blood-brain barrier, are hypo-immunogenic, and carry various “cargos” to their target cell. 1-6 These factors, among many, are the drivers of research to unlock the potential of exosomes.
Exosome Formation
Exosomes are a type of extracellular vesicle, typically between 30-160nm in size that are secreted from multiple cell types (eg, blood cells, endothelial cells, immunocytes, platelets, etc.) and fluids (eg, saliva, blood, cerebrospinal fluid, breast milk, etc). 1 Exosomes are unique from other microvesicles not just because of their small size, but because they contain both intracellular and extracellular components of the source cell. An exosome consists of a protective lipid bilayer1-3 that contains variable cargo, including DNA, mRNA, microRNA (miRNA), cytokines, and cytoplasmic proteins. Exosome production is enhanced under periods of cellular stress7,8 and plays an important role in cell-to-cell communication and immune regulation. 2,5
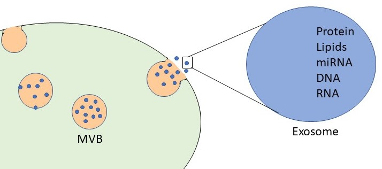
Figure 1. Exosomes are 30-160nm extracellular vesicles containing various cargoes like DNA, mRNA, and proteins.
Adapted from Kalamedits through Wikimedia. Used under the Creative Commons Attribution-Share Alike 4.0 International license.
Exosomes are formed when the cell membrane undergoes endocytosis, creating an endosome. The endosome then creates multiple small vesicles (intraluminal vesicles) by invaginating parts of the endosomal membrane and is now termed a multivesicular body (MVB). Not well understood is how the various “cargos” are sorted into the intraluminal vesicles from the trans-Golgi network, endoplasmic reticulum, and other organelles.9,10 The intraluminal vesicle can include nucleic acids, proteins, lipids, cytokines, transcription factor receptors, mRNA, DNA, and miRNA.2,3,5,11 Not all intraluminal vesicles are released as exosomes; some MVBs may fuse with lysosomes or autophagosomes to be degraded. However, if the MVB fuses with the cell membrane and releases the intraluminal vesicles, those vesicles are now termed “exosomes.” An interesting area of research regarding exosomes has been the possibility of their use in “liquid biopsies.” Because of the vast availability of exosomes in all bodily fluids, testing the composition of the cargo (liquid biopsies) may aid in the diagnosis and prognosis of various cancers and diseases. Research is ongoing regarding using exosomes as markers for glioblastoma, kidney disease, and lung and pancreatic cancers.12,13
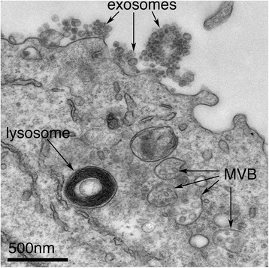
Figure 2. Exosomes correspond to intraluminal vesicles of multivesicular bodies. A transmission electron micrograph of an Epstein–Barr virus-transformed B cell displaying newly expelled exosomes at the plasma membrane. MVB can be seen, which can deliver content to lysosomes for degradation or can fuse with the cell surface to release intraluminal vesicles as exosomes, which is indicated by the arrows at the top of the figure.
Adapted from James R. Edgar. https://dx.doi.org/10.1186%2Fs12915-016-0268-z. Used under the Creative Commons Attribution-Share Alike 4.0 International License.
Exosome Cell Interactions
Exosome-mediated cell communication can occur via several mechanisms, including:
- Internalization of the exosome within the target cell - Intracellular cargo can consist of genes, miRNA, proteins, etc, which then cause a change in the target cell function if released. These changes can impact immune response, tumor progression, neurologic and bone health, and tissue growth. In the context of regenerative medicine, exosomal cargo can induce cartilage repair in animal models and provide a cell-free therapy for OA.4 In some cases, the exosome may be internalized and degraded without any release of the cargo.3,11
- Direct Interaction with target cell surface receptors - The exosomal membrane that was derived from the MVB of the donor cell has a unique combination of ligands, proteins (including heat shock and tetraspanin proteins), ceramides, and cholesterol that can interact with multiple cell-surface receptors on the target cell.1,14 This interaction can stimulate those receptors causing downstream changes.
- Fusion with target cell membrane - Exosomes have a long-circulating half-life and can easily penetrate or fuse with cellular membranes.1 The binding of the exosome to the target cell membrane results in a “new” cell membrane that now has surface receptors from both the donor and target cell,3 affecting target cell interactions with the extracellular environment.
Proper creation is essential since the therapeutic effect of the exosome is wholly dependent on the donor cell sources, culture methods, and isolation techniques.
Exosomes as Therapy
Several of exosomes’ features make them an exciting avenue of research in various therapies. The exosome lipid bilayer acts as a protective mechanism for less stable components like miRNA, enhancing the bioavailability of the cargo. They can cross the blood-brain barrier and capillary border without some of the limitations of cell-based therapies and are small enough to evade being cleared by the reticuloendothelial system.15 These qualities allow exosomes to regulate both regional cellular behavior and more distant immune function, changing immune responses to viral pathogenicity, cardiovascular disease, and cancer progression; these exosome-driven changes can be either disease-altering or promoting.2 Because almost all cells create and release exosomes, the source of these small vesicles is critical in defining their function (ie, exosomes taken from joints with rheumatoid arthritis increase pro-inflammatory cytokines while cancer exosomes stimulate tumor creation). This means that exosomes have the potential to harm as well as heal.
The most common and well-studied exosomes are isolated from cell cultures. There are no standardized methods in exosome concentration, but it is a time and resource-intensive process commonly employing methods such as ultracentrifugation, filtration, polymer precipitation, size exclusion-chromatography, and immunoaffinity capture.3 Harvesting/concentrating exosomes on a commercial scale is one of the major limiting factors to the widespread use of this therapy, and research is ongoing on how to properly scale its production. Proper creation is essential since the therapeutic effect of the exosome is wholly dependent on the donor cell sources, culture methods, and isolation techniques. These variables define if exosome preparations are therapeutic or deleterious.
When properly created, there is a vast array of possible therapies for exosomes. Among those are intracellular delivery of treatments, regenerative medicine, and liquid biopsies. The intracellular delivery of drugs, genes, or proteins to “reprogram” target cells via exosomes has shown promise. Because exosomes contain transmembrane and membrane-anchored proteins that assist with absorption and endocytosis, they represent a significant advantage over current liposomal or synthetic polymer-based drug carriers.3 Another future role of exosomes is their cell regulatory capability, which has produced significant interest for regenerative medicine practitioners. For instance, it has been shown that exosomes from bone marrow stem cells may be as beneficial as the cellular components themselves in improving cartilage and nerve function.16
One of the reasons that exosomes have gained so much attention is their interactions with miRNAs and DNA. This is largely due to the regulatory roles that miRNA plays in gene expression.2 Originally it was thought that miRNA incorporation into exosomes was random, but we now know that miRNAs are preferentially sorted into exosomes11 and are capable of intracellular transfer of these epigenetically active nucleic acids.17 Exosome miRNAs have been implicated in many exosome-mediated cellular activities including angiogenesis, immunomodulation, anti-apoptosis, and anti-fibrosis.17 For example, in a study by Li et al, mesenchymal stem cell exosomal miR-181c was found to significantly reduce burn-induced inflammation in rats.18
Exosomes in Regenerative Medicine
Exosomes may be derived from multiple biologic therapies, especially incubated whole blood or mesenchymal cell cultures (MSCs). They can be extracted from the secretomes of both autologous and allogeneic products. Exosome products are cell-free and hypo-immunogenic due to the lack of MHC-II surface markers and low expression of MHC-I (when compared to their parent’s cells). These qualities greatly increase the future possibilities for allogeneic products.4,6 A study by Yu et al showed that MSC-derived exosomes promote the proliferation, migration, and differentiation of tendon stem/progenitor cells in an animal model of tendinopathy19 Exosome-transported miRNAs have also been shown to promote muscle regeneration via angiogenesis and myogenesis. 20 Additionally, exosomes from MSCs may be a therapy option to treat liver cancer and can promote liver regeneration.14
However, exosomes are not universally therapeutic. When exosomes are isolated from synoviocytes in patients with OA, they increase the secretion of inflammatory cytokines.4 Similarly, exosomes from senescent cells can inhibit cartilage formation when applied to non-senescent cells—essentially transferring OA phenotypes to the healthy cells and creating OA.4,21 On the other hand, exosomes derived from the appropriate sources appear to offer therapeutic benefits and potential tissue repair. They can modulate inflammation and have been shown to restore cartilage health.4,16 The source and the incubation techniques define the function of the exosome.
Future Regulatory Considerations and Points of Consideration
It is important to remember that exosomes are currently not approved by the Food and Drug Administration (FDA) to treat or diagnose any disease, 22 and their use should be limited to FDA biologics license applications or clinical studies. In 2020, the FDA issued a consumer alert regarding the potential risks and unsubstantiated claims of unregulated exosome therapies.23
The FDA is informing the public, especially patients, health care practitioners, and clinics, of multiple recent reports of serious adverse events experienced by patients in Nebraska who were treated with unapproved products marketed as containing exosomes. There are currently no FDA-approved exosome products. Certain clinics across the country, including some that manufacture or market violative ‘stem cell’ products are now also offering exosome products to patients. They deceive patients with unsubstantiated claims about the potential for these products to prevent, treat, or cure various diseases or conditions.22-23
Conclusion
In summary, exosome-based therapies are a dynamic and growing area of research that has the potential to disrupt multiple areas of medicine including drug delivery, regenerative therapies, and diagnosis techniques. Exosome function depends wholly upon the source of the donor cells and can be either constructive or destructive. Exosome research in regenerative medicine, especially in the context of incubated cellular preparations, holds promise as a disease-modifying therapy in the treatment of orthopedic conditions. However, it is important to remember that exosome therapies are not currently FDA-approved, and their use should be limited to research protocols or FDA Biologics License Applications. Further research, standardization, and economies of scale need to be developed before exosomes will be ready for widespread adoption.
Aaron Hanydu-Deutmeyer, DO is the associate program director for the pain medicine fellowship program at Ochsner in New Orleans, LA.
Thomas Buchheit, MD, is the director of Regenerative Pain Therapies at the Center for Translational Pain Medicine in the department of Anesthesiology at Duke University and Durham Veterans Affairs Health Care System in Durham, NC.
References
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019;8(7):727. https://doi.org/10.3390/cells8070727
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367(6478):eaau6977. https://doi.org/10.1126/science.aau6977
- Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomedicine 2020;15:9355-71. https://doi.org/10.2147/IJN.S281890
- Song H, Zhao J, Cheng J, et al. Extracellular vesicles in chondrogenesis and cartilage regeneration. J Cell Mol Med 2021;25(11):4883-92. https://doi.org/10.1111/jcmm.16290
- Zhang Y, Bi J, Huang J, et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine 2020;15:6917-34. https://doi.org/10.2147/IJN.S264498
- Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005;12(1):47-57. https://doi.org/10.1007/s11373-004-8183-7
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018;75(2):193-208. https://doi.org/10.1007/s00018-017-2595-9
- Lehmann BD, Paine MS, Brooks AM, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res 2008;68(19):7864-71. https://doi.org/10.1158/0008-5472.CAN-07-6538
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020;367(6478):eaau6977. https://doi.org/10.1126/science.aau6977
- Kanemoto S, Nitani R, Murakami T, et al. Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem Biophys Res Commun 2016;480(2):166-172. https://doi.org/10.1016/j.bbrc.2016.10.019
- Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 2015;13(1):17-24. https://doi.org/10.1016/j.gpb.2015.02.001
- Sandfeld-Paulsen B, Aggerholm-Pedersen N, Bæk R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol 2016;10(10):1595-602. https://doi.org/10.1016/j.molonc.2016.10.003
- Giusti I, Di Francesco M, Dolo V. Extracellular vesicles in glioblastoma: role in biological processes and in therapeutic applications. Curr Cancer Drug Targets 2017;17(3):221-35 https://doi.org/10.2174/1568009616666160813182959
- Yu X, Odenthal M, Fries JW. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci 2016;17(12):2028. https://doi.org/10.3390/ijms17122028.
- Baharlooi H, Azimi M, Salehi Z, et al. Mesenchymal stem cell-derived exosomes: a promising therapeutic ace card to address autoimmune diseases. Int J Stem Cells 2020;13(1):13-23. https://doi.org/10.15283/ijsc19108
- Zhang S, Chu WC, Lai RC, et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage 2016;24:2135-40. https://doi.org/10.1016/j.joca.2016.06.022
- Toh WS, Lai RC, Zhang B, et al. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans 2018;46(4):843-53. https://doi.org/10.1042/BST20180079
- Li X, Liu L, Yang J, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016;8:72-82. https://doi.org/10.1016/j.ebiom.2016.04.030
- Yu H, Cheng J, Shi W, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater 2020;106:328-41. https://doi.org/10.1016/j.actbio.2020.01.051
- Nakamura Y, Miyaki S, Ishitobi H, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 2015;589(11):1257-65. https://doi.org/10.1016/j.febslet.2015.03.031
- Jeon OH, Wilson DR, Clement CC, et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019;4(7):e125019
- Consumer alert on regenerative medicine products including stem cells and exosomes. U.S. Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/consumer-alert-regenerative-medicine-products-including-stem-cells-and-exosomes. Published July 22, 2020.
- Public safety notification on exosome products. U.S. Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-exosome-products. Published December 6, 2019.

