A Review of Pain Management in the Intensive Care Unit
Pain in critically ill patients is often underdiagnosed and undertreated. In this population, there are many potential barriers to pain recognition and management. Untreated and undertreated pain is distressing for patients, family members, and caregivers; in addition, neglected pain may contribute to increased morbidity and mortality. Assessment of pain in the intensive care unit (ICU) can be difficult; many critically ill patients cannot communicate their discomfort because of intubation, sedation, or cognitive impairment. However, in its “Clinical Practice Guidelines for the Management of Pain, Agitation and Delirium in Adult Patients in the Intensive Care Unit,” the Society of Critical Care Medicine (SCCM) recommends that pain be routinely monitored in all adult ICU patients. Unfortunately, it is difficult to estimate the incidence of pain in critically ill patients because pain assessment tools and protocols for the management of pain are rarely applied. A Canadian study of 51 ICUs found that less than 20% of ICUs used pain assessment tools and only 25% of ICUs used pain protocols. A separate multicenter observational study found that 90% of patients in the ICU were being actively treated with opioids whereas only 42% had undergone a pain assessment. Similarly, Payen et al reported that pain was not assessed in 53% of patients who were receiving analgesia, and when pain was assessed, specific pain tools were used only 28% of the time.[1–3]
“It is difficult to estimate the incidence of pain in critically ill patients because pain assessment tools and protocols for the management of pain are rarely applied.”
However, studies have aimed to quantify the incidence of pain in critically ill patients. We know from prospective descriptive studies that the presence of an endotracheal tube has been reported as a constant source of discomfort at rest and that routine procedures—such as tracheal suctioning, position changes, and line removal—cause pain.[4] One study suggested that pain is frequent with an incidence of 50% in medical and surgical patients at rest and 80% during common care procedures.[5] Another study showed similar results when patients recently discharged from the ICU were interviewed about their pain during hospitalization. Nearly 50% of patients reported recall of pain during their ICU stay. Fifteen percent of ICU patients reported extremely severe pain or moderately severe pain occurring at least half the time. Not surprisingly, nearly 15% of patients were dissatisfied with pain control during their ICU stay.[6] Another study showed that 63% of patients received no analgesics before or during painful procedures.[7]
Why Should We Care?
In the article “Pain Management: A Fundamental Human Right,” Brenan et al wrote, “Unreasonable failure to treat pain is viewed worldwide as poor medicine, unethical practice, and an abrogation of a fundamental human right.”[8] FaberLangendoen et al wrote, “Many believe the obligation of clinicians to tend to patients' suffering is the essence of the medical profession.” In addition to the ethics of pain management, medical outcomes are improved when pain is optimally managed.[9]
Pain assessment in patients on mechanical ventilation has been independently associated with a decrease in hypnotic drug dosing, duration of mechanical ventilation, and duration of ICU stay.[10] Pain contributes to hypoventilation and reduced cough, which increases atelectasis and sputum retention. These mechanisms are thought to be responsible for the increased rate of ventilator-associated pneumonia (VAP) in patients who are not routinely assessed for pain. Payen et al demonstrated decreased risk of VAP in patients routinely assessed and treated for pain.[10] Chanques et al validated those findings when they reported significantly decreased risk of VAP and duration of mechanical ventilation when pain was routinely assessed and treated.[11] Without using validated pain assessment tools and protocols, patients in the ICU are often managed inappropriately with sedation medications. Continuous sedation, titrated to a light level and with daily sedation interruptions, has been associated with an increased duration of mechanical ventilation and ICU length of stay when compared to sedation-free protocols.
Less sedative medication allows for early mobilization, which in turn results in improved outcomes. In studies designed to compare standard of care versus early mobilization of ventilated patients, patients who were randomized to early mobilization had shorter ICU and hospital lengths of stay and were less likely to die or be rehospitalized in the year following their critical illness.[12-13]
Other potential acute negative effects of untreated pain include delirium, self-harm from accidental removal of lines or tubes, sympathetic activation with increased catecholamine release leading to tachycardia and increased systemic vascular resistance, increased cardiac workload leading to oxygen supply demand mismatch, and myocardial ischemia.[14-15] In addition to acute negative health effects, untreated and undertreated pain has been associated with the development of chronic medical issues, including chronic pain, long-term psychological illness, and lower quality of life.[16-17]
How Should We Assess Pain in the ICU?
Two sensitive and validated measures are used to assess pain in patients unable to communicate their pain: the Critical Care Pain Observation Tool (CPOT) and the Behavioral Pain Score (BPS).
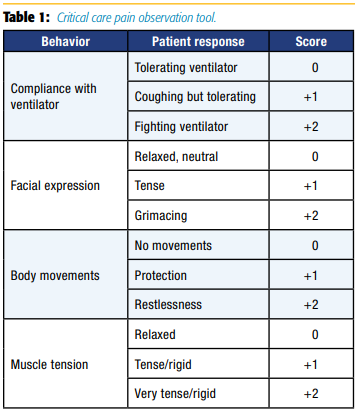
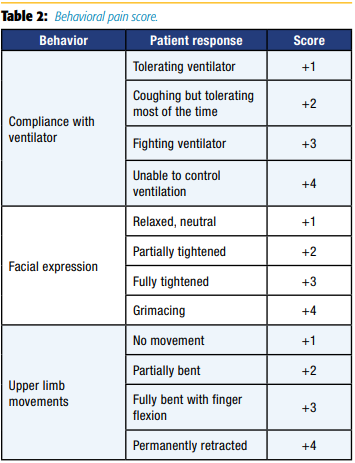
CPOT evaluates four behaviors—facial expressions, body movements, muscle tension, and compliance with the ventilator for mechanically ventilated patients or vocalization for nonintubated patients—rated on a scale of 0–2 with a total score ranging from 0–8 (see Table 1).[18]
BPS evaluates three behaviors—facial expressions, upper limb movements, and ventilator compliance—rated on a scale of 1–4 with a total score ranging from 3–12 (see Table 2).[19]
The SCCM makes numerous recommendations about treating pain in the ICU. They acknowledge the presence of pain at rest and in routine care, and they make recommendations that pain be routinely monitored using BPS or CPOT for patients who are unable to self-report.[20] They emphasize that validated scoring systems should be used to assess pain and state that changes in blood pressure and tachycardia should not be routinely used as measures for assessment of pain.[21] For more information regarding the SCCM recommendations, refer to the SCCM pain, agitation, and delirium guidelines as well as the ABCDEF bundle for prevention of postintensive care syndrome.
Aside from difficult assessment of pain in the critically ill patient, there are many other obstacles to pain management in the ICU patient.
- Impaired Renal and/or Hepatic Clearance
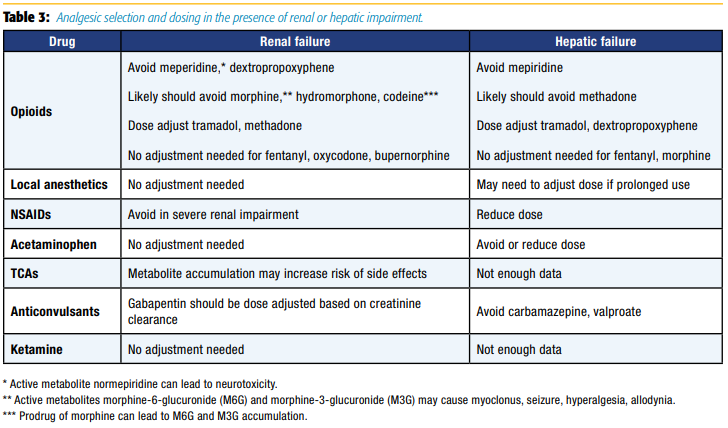
Critically ill patients often have organ failure with associated decreases in renal or hepatic clearance; thus, drug choice and dosing should be carefully considered. Table 3 reviews considerations of analgesic selection and dosing in the presence of renal or hepatic impairment.
- Hemodynamic Instability
Patients in ICUs are often hemodynamically unstable. Hypotension after the use of opioids is generally due to blunting of sympathetic responses and may unmask hypotension. For this reason, bolus doses should be administered slowly, and short-acting opioids are preferred.
- Obstacles to Regional Anesthesia
Regional anesthesia may be considered as an adjunct to decrease opioid consumption in the critically ill surgical patient. However, coagulopathy of the critically ill and anticoagulant medications should be considered carefully prior to the implementation of regional anesthesia.[22] In addition, systemic infection and positioning challenges (eg, fractures and an inability to cooperate) may preclude safe neuraxial or peripheral nerve blockade. The SCCM makes no recommendation for neuraxial/regional analgesia over systemic analgesia in medical ICU patients due to lack of evidence, but they do acknowledge thoracic epidural superiority over parenteral opioids for abdominal aortic surgery.[20]
- Pharmacologic Side Effects
Drug side effects may slow recovery, worsen patient outcomes, or create new issues. Opioids can contribute to ileus, delirium, and respiratory depression. It is generally accepted that patients with long-term exposure to high-dose opiates may develop physiologic dependence.
Intravenous opioids are first-line therapy for non-neuropathic pain. Opioids may be administered by the patient's RN on a scheduled or as-needed basis, but they may also be administered using patientcontrolled analgesia (PCA), in which the patient is given the ability to self-administer pain medication. Any opioid can be administered by PCA pumps; however, meperidine is not recommended for repeat dosing because it lowers the seizure threshold and has a dysphoric effect.[23] In general, basal infusions are not recommended, but they may be appropriate for opioid-tolerant patients and select patients in the ICU. PCA may not be appropriate for a substantial portion of the intensive care population because of underlying disease processes and the systemic effects it may have on cognition.[24]
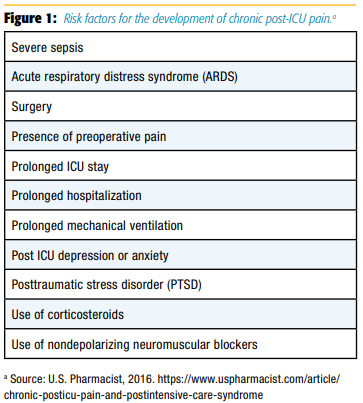
It is now recognized that long-term survivors of medical and ICUs are at high risk for developing chronic pain syndromes.[25] Risk factors for development of chronic post-ICU pain are described in Figure 1. [26]
Patients at high risk for neuropathic pain—for example, Guillain-Barre syndrome, burns, amputations, and spinal cord injury— should be considered for early administration of gabapentin and carbamazepine; however, these medications have not been shown to be consistently effective.[20],[27] Burn patients receiving dressing changes should be treated with fast-acting opioids, anxiolytics, and ketamine to decrease anticipatory anxiety and development of post-traumatic stress disorder and chronic pain.[28]
Although opioids are considered first-line therapy for nonneuropathic pain, a multimodal approach to pain management may help decrease opioid requirements and thus the side effects of opioid use. Nonsteroidal anti-inflammatory drugs are effective but may be contraindicated because of the risks of gastric ulcers, bleeding, and renal dysfunction. Nonpharmacologic interventions for pain management (music therapy, relaxation) should be considered because they are generally low risk, low cost, and safe; unfortunately, there is a lack of evidence to make a strong recommendation for use.
Palliative and end-of-life pain management is also an important concern for physicians in intensive care units because 20% of patients who die in the hospital report pain and 50% of hospice patients report daily pain. Alleviation of dyspnea and pain should be the goal of drug therapies.[11]
In summary, critically ill patients routinely experience pain and are often not able to communicate this to their healthcare team. Undertreated pain can contribute negatively to both short- and long-term outcomes. Pain should be routinely assessed and treated in critically ill patients using validated assessment tools such as CPOT and BPS.
References
- Payen J-F, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 2007;106(4):687–695. doi: 10.1097/01.anes.0000264747.09017.da
- Burry LD, Williamson DR, Perreault MM, et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Canadian Journal of Anesthesia 2014;61(7):619–630. doi: 10.1007/s12630-014-0174-1
- Kumar AB, Brennan TJ. Pain assessment, sedation, and analgesic administration in the intensive care unit. Anesthesiology 2009;111(6):1187–1188. doi: 10.1097/ALN.0b013e3181c0d1b5
- Siffleet J, Young J, Nikoletti S, Shaw T. Patients' self-report of procedural pain in the intensive care unit. J Clin Nurs 2007;16(11):2142–2148. doi: 10.1111/j.1365-2702.2006.01840.x
- Chanques G, Sebbane M, Barbotte E, Viel E, Eledjam JJ, Jaber S. A prospective study of pain at rest: incidence and characteristics of an unrecognized symptom in surgical and trauma versus medical intensive care unit patients. Anesthesiology 2007;107:858–860. doi: 10.1097/01. anes.0000287211.98642.51
- Desbiens NA, Wu AW, Broste SK, et al. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. Crit Care Med 1996;24:1953–1961.
- Puntillo KA, Wild LR, Morris AB, et al. Practices and predictors of analgesic interventions for adults undergoing painful procedures. Am J Crit Care 2002;11(5):415–429.
- Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg 2007;105:205–221. doi: 10.1213/01.ane.0000268145.52345.55
- Faber-Langendoen K, Chao T. Pain control. In: 20 Common Problems: Ethics in Primary Care. Sugarman J (ed.). New York, New York: McGraw-Hill; 2000:199–210.
- Payen JF, Bosson JL, Chanques G, Mantz J, Labarere J. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology 2009;111(6):1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0
- Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56
- Morris PE, Griffin L, Berry M, et al. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci 2011;341:373–377. doi: 10.1097/ MAJ.0b013e31820ab4f6
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. doi: 10.1016/S0140- 6736(09)60658-9
- Reade M, Phil D, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med 2014;370:444–454. doi: 10.1056/NEJMra1208705
- Page GG, Blakely WP, Ben-E liyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 2001;90:191– 199.
- Schelling G, Stoll C, Haller M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med 1998;26:651–659.
- Myhren H, Ekeberg O, Toien K, Karlsson S, Stokland O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care 2010;14:R14–R14. doi: 10.1186/cc8870
- Gélinas C, Fortier M, Viens C, Fillion L, Puntillo KA. Pain assessment and management in critically ill intubated patients: a retrospective study. Am J Crit Care 2004;13:126–135.
- Payen JF, Bru O, Bosson JL, et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001;29(12):2258–2263.
- Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41(1):263–306. doi: 10.1097/ CCM.0b013e3182783b72
- Young J, Siffleet J, Nikoletti S, et al. Use of a behavioural pain scale to assess pain in ventilated, unconscious and/or sedated patients. Intensive Crit Care Nurs 2006;22:32–39. doi: 10.1016/j.iccn.2005.04.004
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (3rd ed.). Reg Anesth Pain Med 2010;35:64–101.
- Latta KS, Ginsberg B, Barkin RL. Meperidine: a critical review. Am J Ther 2002;9(1):53–68.
- Grass JA. Patient-controlled analgesia. Anesth Analg 2005;101:S44–S61.
- Baumbach P, Gotz T, Gunther A. Prevalence and characteristics of chronic intensive care–related pain: the role of severe sepsis and septic shock. Crit Care Med 2016;44(6):1129–1137. doi: 10.1097/CCM.0000000000001635
- Johnson J, Al-Dahir S. Chronic post-ICU pain and post-intensive care syndrome. US Pharm 2016;41(3):HS11–HS15. https://www.uspharmacist.com/article/ chronic-posticu-pain-and-postintensive-care-syndrome. Accessed September 9, 2017.
- Pena L, Moreno CB, Gutierrez-Alvarez AM. Pain management in Guillain-Barre syndrome: a systematic review. Neurologia 2015;30(7):433–438. doi: 10.1016/j. nrl.2014.04.009
- Upton D, Andrews A. The impact of stress at dressing change in patients with burns: a review of the literature on pain and itching. Wounds 2014;26:77–82.
Leave a commentOrder by
Newest on top Oldest on top