Rebound Pain After a Nerve Block Wears Off
The benefits of regional anesthesia are well known and include reduced postoperative pain, decreased perioperative opioid administration, and improved patient satisfaction. Following block resolution, rebound pain has been recently recognized as an important complication related to the administration of peripheral nerve blocks.[1]
Rebound pain is the quantifiable difference in pain scores when a peripheral nerve block is working versus the acute pain that is encountered when the block wears off.[2],[3] Rebound pain is often considered out of proportion to the degree of the surgical stimulus, and it may lead to an increased use of opioid analgesics and decreased patient satisfaction, thus undermining the perceived and real success and benefits associated with regional anesthesia.[4] This article aims to describe the phenomenon of rebound pain, its prevalence in the literature, and a suggested standard for reporting. In addition, potential etiologies and strategies to prevent and/or minimize rebound pain severity will be presented.
“Handling these intensely painful moments, especially with the potential for occurring outside of a health care setting, constitutes a clinically relevant problem.”
A recent qualitative study involving ankle surgery found that rebound pain following block resolution is a real concern to patients.[5] Although the patients appreciated the mental alertness and analgesia that the nerve blocks provided, they experienced difficulty predicting their analgesic needs following dissipation of these blocks and how to effectively prevent or manage rebound pain. As such, handling these intensely painful moments, especially with the potential for occurring outside of a health care setting, constitutes a clinically relevant problem.[5]
Rebound pain occurs across a variety of surgeries, nerve blocks, and local anesthetics and has been corroborated in animal models. A 2015 meta-analysis found that rebound pain may render single-shot interscalene blockade less beneficial to patients undergoing ambulatory shoulder surgery than previously believed.[6] The patients had improved pain control up to 8 hours and an opioid-sparing effect up to 12 hours following surgery as compared to those receiving no block. However, the patients who received the block reported increased pain 16 hours postoperatively (1.16 on a 0–10 pain scale, 99% confidence interval [CI]: 0.02–2.30, p = .009), with no difference beyond 24 hours. The rebound pain as the block wore off was consistent across the meta-analysis, regardless of local anesthetic type, volume, or concentration used.6 With the exception of epinephrine, adjuvants capable of prolonging the single-shot block were excluded from this study.[7]
In patients undergoing anterior cruciate ligament reconstruction, single-shot femoral nerve blockade resolution led to an associated acute increase in pain scores (2.0 on a 0–10 pain scale, 95% CI: 1.6–2.4, p < .05). Although increased nerve block duration (ie, femoral catheter infusion vs single-shot block) reduced the severity of rebound pain, it did so at a clinically insignificant rate: 0.03 units on a 0–10 pain scale per hour of block duration (95% CI: 0.02–0.05, p < .001).2 In other words, 33 hours of a nerve block duration difference led to a rebound pain reduction of 1 (on a 0–10 scale). Other studies have shown that certain regional techniques and surgeries pose a higher risk for causing rebound pain. Following total knee arthroplasty, periarticular injections provided greater immediate analgesia, yet more rebound pain, than did femoral nerve blocks or the combination of both blocks.[8] Unpublished data from our group also showed that associated rebound pain was higher after shoulder surgery than after complex knee surgery.[2]
Rebound pain has also been demonstrated in animal models, although the clinical relevance of these findings is uncertain. In one study, rats that underwent sciatic nerve blockade with ropivacaine were found to have transient hyperalgesia to heat stimuli (but not mechanical stimuli) as the block wore off (at 3 hours) as compared to rats receiving placebo blocks, suggesting a potential nerve fiber specificity in the pathophysiology of rebound pain.9 Transient heat hyperalgesia was also found in a separate rat study at the 4-hour mark.10 In rat studies, these interval findings regarding nerve block duration and rebound pain could translate to hours of comfort then discomfort for our patients. With the increased attention and research that has evaluated rebound pain, several patterns have become apparent. Typically, rebound pain occurs 16–24 hours postoperatively, is experienced commonly at night, and negatively affects quality of sleep. Patients also commonly describe rebound pain as a burning sensation. Rebound pain can profoundly impact the patient recovery experience and ultimately affect overall opioid consumption, emergency department visits, and patient satisfaction.[5],[11]
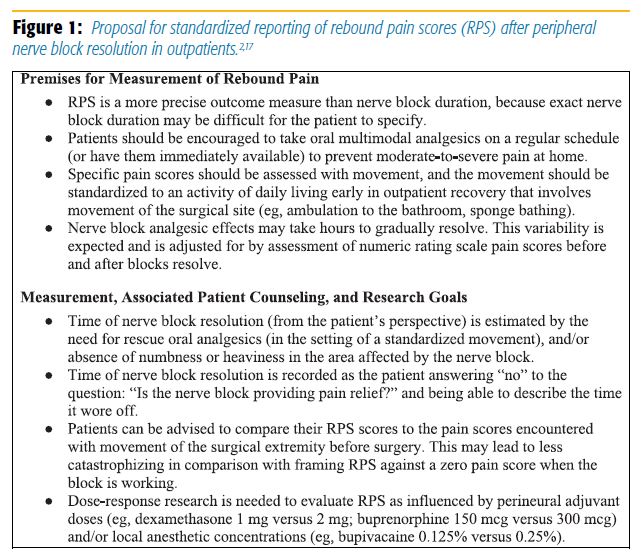
To better quantify this problem, Williams and colleagues[2] have proposed a standardized method of reporting rebound pain scores (Figure 1). Given the clinical implications, it is necessary to understand the pathophysiology of rebound pain as more than just the resolution of the nerve block. Although early animal models have pointed to potential modality- and nociceptor-specific mechanisms, other potential processes have emerged.[9]
Local anesthetics are widely known to be neurotoxic and may be the source of neuronal damage or altered conduction that could manifest as rebound pain.[12] Another theory proposes that although nerve blockade prevents signal transduction, nociceptive signal memories are retained and amplified when the block ultimately wears off.[11] Patient noncompliance or confusion can lead to poor transitioning from nerve block to oral analgesics.[13] Finally, patients may have catastrophizing misconceptions about the block, worry about permanent nerve damage, or develop a falsely low pain tolerance.[5] Acute opioid-induced hyperalgesia (ie, from either opioids used in the operating room or before the block wears off) is another hypothesis that we introduce here.
Regardless of cause, potential methods may minimize the effect of rebound pain on patients undergoing peripheral nerve blockade. Regional anesthesiologists can supplement their blocks with preoperative, intraoperative, and postoperative multimodal-enhanced, recovery-driven protocols with oral and intravenous analgesics.11 This method may help cover the transition period as the nerve block wears off. Additionally, if local anesthetic concentrations are what drive the severity of rebound pain in vivo (yet unstudied) for blocks that are combined with a general anesthetic and intended to be used more for analgesia than surgical anesthesia, lower local anesthetic concentrations could be used in combination with perineural adjuvants.[14] When general anesthesia is required, two strategies are avoiding use of hyperalgesic agents such as volatile gases and short-acting opioids and including use of agents that modulate the pain response such as esmolol.[15],[16]
Rebound pain can also be theoretically attenuated by prolonging the duration of the block, whether through the use of a continuous infusion via peripheral catheter or long-acting, single-shot injectates. In theory, this could reduce the inflammation and retained neuronal memories associated with surgery. However, as previously discussed, the duration of analgesia must be significantly longer than a typical single-shot block to become clinically effective in reducing rebound pain.[2],[11] Unfortunately, the expected rehabilitation plan and patient preferences may not allow for an extended period of motor blockade or diminished sensation.
Adjuvants may also play a role in decreasing rebound pain. Such drugs not only prolong the duration of the local anesthetic but also may modulate the block in a way that decreases rebound pain through other unknown mechanisms. A recent case series of perineural adjuvants found that a combination of clonidine, buprenorphine, and dexamethasone was associated with reduced severity of rebound pain when administered with bupivacaine or ropivacaine, although optimal dosing is still unclear and varies with extremity.[14,17] Specifically, more buprenorphine was associated with less rebound pain, whereas less dexamethasone was paradoxically associated with less rebound pain. In this case series, however, rebound pain was compared with preoperative pain scores with movement, which may prove to be a more useful parameter than when the block is fully functioning.[17]
In the end, educating patients may prove the most useful short-term management strategy. Including rebound pain during the risk–benefit discussion during the preoperative assessment can inform patients regarding what to expect when the block wears off. Baseline pain evaluations and psychological counseling for high-risk patients may be beneficial.[3] Patients and caregivers can also be instructed to stay ahead of the pain by taking long-acting analgesics while the block is still working.[11] Common formulations such as gabapentin, acetaminophen, ibuprofen, and dextromethorphan may prove useful as rescue medications for rebound pain.
Although the concept of rebound pain requires consideration and discussion, patients still express overall satisfaction from their nerve block experience.[5] Therefore, the multiple benefits of regional anesthesia make this a technique that should continue to be offered until further research can provide more clarity.
References
- Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks inthe management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–529.
- Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. 2007;32(3):186–192.
- Williams BA. Forecast for perineural analgesia procedures for ambulatory surgery of the knee, foot, and ankle: applying patient-centered paradigm shifts. Int Anesthesiol Clin. 2012;50(1):126–142.
- Borgeat A. Single-shot interscalene block: light and shadows. Anesth Analg. 2015;120(5):995–996.
- Henningsen MJ, Sort R, Møller AM, Herling SF. Peripheral nerve block in ankle fracture surgery: a qualitative study of patients' experiences. Anaesthesia. 2018;73(1):49–58.
- Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up: a systematic review and meta-analysis. Anesth Analg. 2015;120(5):1114–1129.
- Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015;10(9):e0137312.
- Youm YS, Cho SD, Cho HY, Hwang CH, Jung SH, Kim KH. Preemptive femoral nerve block could reduce the rebound pain after periarticular injection in total knee arthroplasty. J Arthroplasty. 2016;31(8):1722–1726.
- Kolarczyk LM, Williams BA. Transient heat hyperalgesia during resolution
of ropivacaine sciatic nerve block in the rat. Reg Anesth Pain Med.
2011;36(3):220–224. - Janda A, Lydic R, Welch KB, Brummett CM. Thermal hyperalgesia after sciatic nerve block in rat is transient and clinically insignificant. Reg Anesth Pain Med. 2013;38(2):151–154.
- Abdallah FW. What happens when the blocks wear off: strategies for rebound pain after single-shot blocks. Paper presented at: 14th Annual Symposium on Regional Anesthesia, Pain, and Perioperative Medicine; 2015; New York, NY.
- Verlinde M, Hollmann MW, Stevens MF, Hermanns H, Werdehausen R, Lirk P. Local anesthetic-induced neurotoxicity. Int J Mol Sci. 2016;17(3):339.
- Goldstein RY, Montero N, Jain SK, Egol KA, Tejwani NC. Efficacy of popliteal block in postoperative pain control after ankle fracture fixation: a prospective randomized study. J Orthop Trauma. 2012;26(10):557–561.
- Knight JB, Schott NJ, Kentor ML, Williams BA. Neurotoxicity of common peripheral nerve block adjuvants. Curr Opin Anaesthesiol. 2015;28(5):598–604.
- Malik OS, Kaye AD, Urman RD. Perioperative hyperalgesia and associated clinical factors. Curr Pain Headache Rep. 2017;21(1):4.
- Gelineau AM, King MR, Ladha KS, Burns SM, Houle T, Anderson TA. Intraoperative esmolol as an adjunct for perioperative opioid and postoperative pain reduction: a systematic review, meta-analysis, and meta-regression. Anesth Analg.
2018;126(3):1035–1049. - Williams BA, Ibinson JW, Mangione MP, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011–2014). Pain Med. 2015;16(1):7–12.
Leave a commentOrder by
Newest on top Oldest on top