Review of Dexmedetomidine (Precedex) for Acute Pain and Analgesia
Management of acute postoperative pain is an important health care issue.1 Multimodal analgesia is a strategy employing more than one type of analgesic agent or technique, resulting in additive or synergistic analgesia while reducing adverse effects encountered with the sole use of one analgesic.[1],[2] The American Society of Anesthesiologists recommends multimodal analgesia for acute postoperative pain management.[3] As many as 80% of surgical patients experience postoperative pain, with ramifications such as poor patient satisfaction, the development of persistent postsurgical pain, and extended time in the postanesthesia recovery unit.[4-5] Recent literature suggests that administration of dexmedetomidine may play a promising role as a part of multimodal analgesia in the perioperative period.[1-2][4–6]
“Of note, many of the studies have also shown that perioperative dexmedetomidine use has a trend toward decreased adverse side effects such as PONV when compared with other commonly prescribed analgesic medications.”
Dexmedetomidine was first approved by the Food and Drug Administration in 1999 for short-term use as an analgesic and sedative medication in the intensive care unit.[6] Compared with clonidine, the prototypical alpha (α)-2 adrenoreceptor, dexmedetomidine is eight times more specific for α-2 adrenoreceptors with an α2:α1 selectivity ratio of 1,600:1.7 As a highly selective α-2 adrenoreceptor agonist, this medication functions as a sedative, anxiolytic, and analgesic without any respiratory depressive effects. It has a short terminal half-life of 2 hours, compared with 8 hours for clonidine, and its most notable side effects are bradycardia and hypotension, although hypertension is observed only with higher doses.[6–8] Administration routes include intravenous, intramuscular, perineural, intranasal, buccal, epidural, and intrathecal.
Several studies have investigated the potential benefits of dexmedetomidine delivered perioperatively. Review of recent literature finds that most discuss intravenous, perineural, and neuraxial administration and its impact on perioperative outcomes. This article focuses on studies and meta-analyses investigating perioperative dexmedetomidine administration and its effect on analgesia (summarized in Tables 1 and 2).
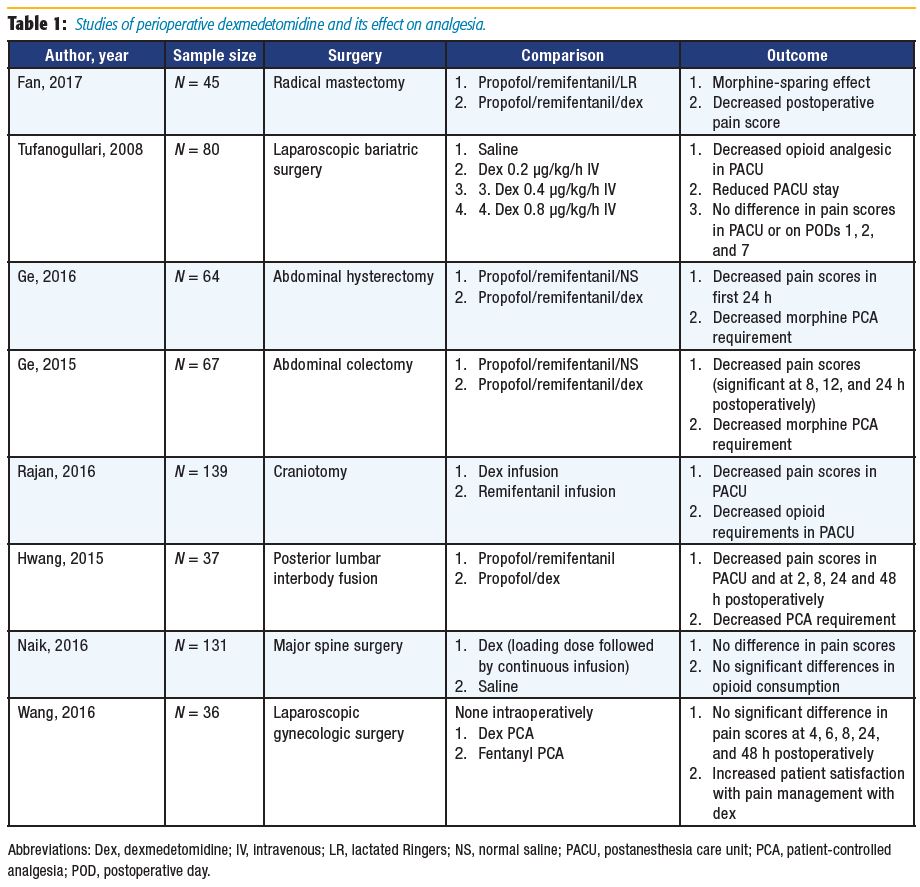
Acute postoperative pain is common among patients undergoing radical mastectomy, which increases the risk of chronic hyperalgesia.[9] Fan et al[9] randomized 45 patients undergoing radical mastectomy to receive intraoperative lactated Ringers or dexmedetomidine. The time to patients’ first morphine request was greater in the dexmedetomidine group. Postoperative patientcontrolled anesthesia (PCA) use in the first 24 hours was also decreased, with lower reported pain scores at rest and with activity compared with the control group. The authors suggested that dexmedetomidine has an opioid-sparing effect, and its use may promote postoperative analgesia.[9]
A number of authors have researched the use of intraoperative dexmedetomidine specifically for patients undergoing abdominal surgeries.10–12 Ge et al[11-12] reported on the primary outcome of postoperative opioid use in the first 24 hours and found reduced consumption, which correlates with results from Tufanogullari et al.[10] However, Tufanogullari et al[10] did not find decreased opioid requirements on postoperative days 1, 2, or 7. Both studies concluded that dexmedetomidine was beneficial in their patient populations, with Tufanogullari et al[10] further recommending a 0.2 μg/kg/h infusion rate to minimize the risk of adverse cardiovascular side effects.
Following general anesthesia for neurosurgical cases, a rapid neurological assessment of patients is frequently desired. Remifentanil is often a popular choice in such cases because it provides rapid and reliable emergence from general anesthesia.[13] Rajan et al[13] and Hwang et al[14] compared remifentanil and dexmedetomidine infusions in patients undergoing craniotomy or posterior lumbar interbody fusion, respectively. The authors concluded that dexmedetomidine was a reasonable alternative to remifentanil because their results demonstrated decreased postoperative opioid consumption with decreased postoperative pain scores.[13-14]
A study by Naik et al[15] compared intraoperative dexmedetomidine to placebo in patients undergoing major spine surgery and failed to demonstrate a statically significant difference between postoperative opioid consumption and pain scores, although the authors found a modest but significant reduction in intraoperative opioid consumption. Wang et al[16] provided a unique study design in which dexmedetomidine was administered as postoperative PCA to patients undergoing laparoscopic gynecologic surgery. Neither the fentanyl PCA nor dexmedetomidine PCA group required rescue analgesia. Patients receiving postoperative dexmedetomidine had higher pain control satisfaction scores, a decreased incidence of postoperative nausea and vomiting (PONV), and faster recovery of bowel function.[16]
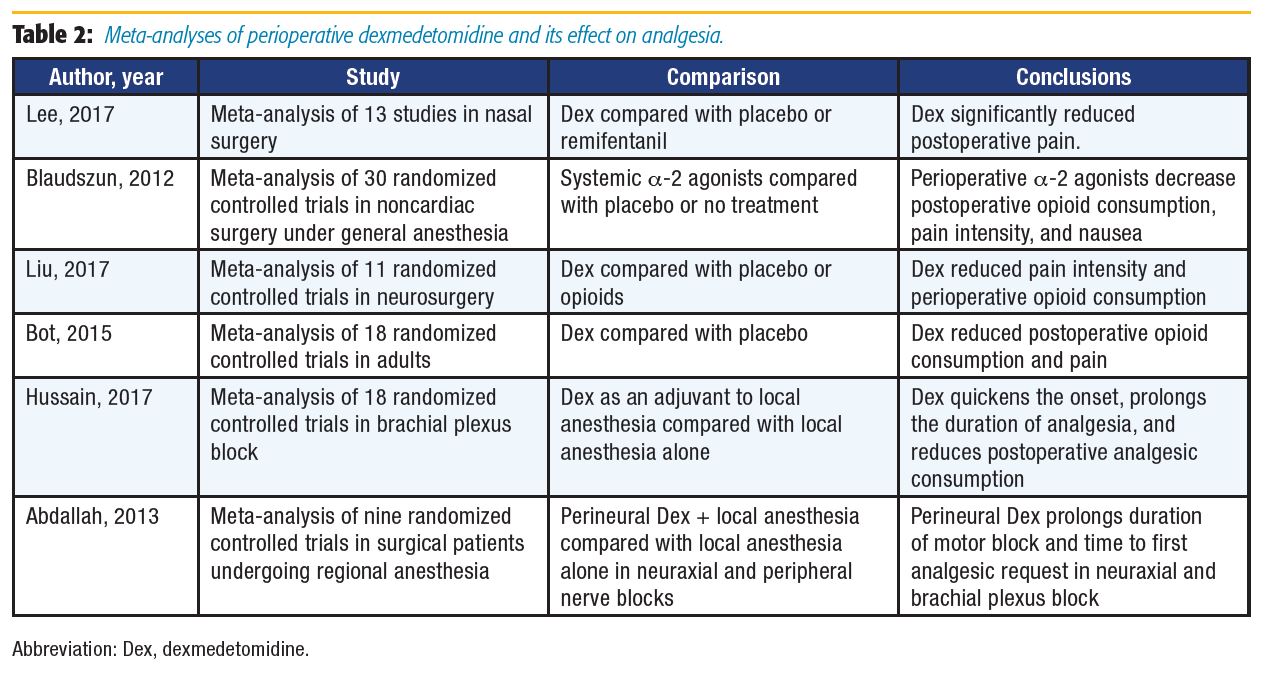
When examining outcomes following nasal surgery, Lee et al[17] found reduced opioid consumption with systemic administration of dexmedetomidine. Dexmedetomidine compared to placebo or no treatment was investigated in three different meta-analyses for various surgical procedures. All analyses found similar results, including decreased opioid consumption and reduced pain or pain intensity in the groups receiving dexmedetomidine.[18–20] Dexmedetomidine has also been used as an adjuvant to local anesthetics (LA). As an intrathecal or epidural adjuvant, it provides better postoperative analgesia,[4,6] and as an adjuvant in peripheral nerve block and intravenous regional anesthesia, it has been reported to shorten onset time, prolong duration of postoperative analgesia, and improve block quality.[6] Recently, Hussain et al[21] reviewed the efficacy of dexmedetomidine as an adjuvant to LA in brachial plexus blocks and found that dexmedetomidine significantly shortened the block onset time by 3.2 minutes, prolonged sensory blockade by 261 minutes, and prolonged the duration of analgesia by 289 minutes.
Abdallah et al[22] examined the effects of perineural dexmedetomidine as an LA adjuvant in neuraxial and brachial plexus blocks compared with LA alone. As in the Hussain et al[21] meta-analysis, dexmedetomidine was found to increase the duration of sensory block in brachial plexus analgesia; however, the difference was not statistically significant. Motor block duration and time to first analgesic request were prolonged in both neuraxial and brachial plexus blocks. The prolongation of motor blockade may offset the benefits of dexmedetomidine if it delays rehabilitation or discharge, especially in the ambulatory setting. Conclusive neurotoxicity data on perineural dexmedetomidine use are lacking: some animal data suggest dexmedetomidine is protective against hypoxic-ischemic neuronal injury, whereas other studies have shown evidence of demyelination in white matter when administered via the epidural route.[6],[22]
The current literature on dexmedetomidine used perioperatively suggests that in certain clinical scenarios, the drug decreases perioperative opioid consumption and improves postoperative analgesia. Of note, many of the studies have also shown that perioperative dexmedetomidine use has a trend toward decreased adverse side effects such as PONV when compared with other commonly prescribed analgesic medications. More research is warranted to establish the efficacy and safety of perioperative dexmedetomidine for the management of acute pain and analgesia, with a closer look at its utility in the postoperative period as well as the ambulatory setting.
References
- Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215– 2225.
- Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593.
- Apfelbaum JL, Ashburn MA, Connis RT, et al. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273.
- Luo J, Min S. Postoperative pain management in the postanesthesia care unit: an update. J Pain Res. 2017;10:2687–2698.
- Helander EM, Menard BL, Harmon CM, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep. 2017;21:3.
- Grewal A. Dexmedetomidine: new avenues. J Anaesthesiol Clin Pharmacol. 2011;27(3):297–302.
- Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005;18:412–418.
- Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–1904.
- Fan W, Xue H, Sun Y, et al. Dexmedetomidine improves postoperative patient-controlled analgesia following radical mastectomy. Front Pharmacol. 2017;8:250.
- Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106:1741–1748.
- Ge DJ, Qi B, Tang G, Li JY. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep. 2016;6:21514.
- Ge DJ, Qi B, Tang G, Li JY. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy. Medicine. 2015;94(43):e1727.
- Rajan S, Hutcherson MT, Sessler DI, et al. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: a randomized controlled trial. J Neurosurg Anesthesiol. 2016;28:282–290.
- Hwang W, Lee J, Park J, Joo J. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol. 2015;15:21.
- Naik BI, Nemergut EC, Kazemi A, et al. The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth Analg. 2016;122:1646–1653.
- Wang X, Liu W, Xu Z, et al. Effect of dexmedetomidine alone for intravenous patient-controlled analgesia after gynecological laparoscopic surgery. Medicine. 2016;95(19):e3639.
- Lee HS, Yoon HY, Jin HJ, Hwang SH. Can dexmedetomidine influence recovery profiles from general anesthesia in nasal surgery? Otolaryngol Head Neck Surg. 2018;158:43–53.
- Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity. Anesthesiology. 2012;116:1312–1322.
- Liu Y, Liang F, Liu X, et al. Dexmedetomidine reduces perioperative opioid consumption and postoperative pain intensity in neurosurgery: a meta-analysis. J Neurosurg Anesthesiol. 2017;30(2):146–155.
- Bot AL, Michelet D, Hilly J, et al. Efficacy of intraoperative dexmedetomidine compared with placebo for surgery in adults: a meta-analysis of published studies. Minerva Anestesiol. 2015;81:1105–1117.
- Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42:184–196.
- Abdallah FW, Brull R. Faciliatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110:915–925.
Leave a commentOrder by
Newest on top Oldest on top