How I Do It: Shoulder Articular Nerve Blockade and Radiofrequency Ablation
Cite as: Eckmann M, Joshi M, Bickelhaupt B. How I do it: shoulder articular nerve blockade and radiofrequency ablation. ASRA News 2020;45. https://doi.org/10.52211/asra110120.062
Introduction
Chronic shoulder joint pain is the third most common musculoskeletal complaint. It is a common and burdensome condition, with an estimated prevalence of 7-26%.[1] The shoulder joint has the most complex range of motion of the major joints. The shoulder girdle, which connects the upper extremity to the thorax, includes four joints or articulations: 1) the glenohumeral joint, 2) the acromioclavicular joint, 3) the sternoclavicular joint, and 4) the scapulothroacic articulation. The bones that comprise these structures include the humerus, scapula, and clavicle, and the joint also relies on dynamic stabilization from the muscles of the rotator cuff (supraspinatus, infraspinatus, teres minor, subscapularis). Hilton’s law of joint innervation, which has been validated[2] and has stood the test of time since 1863, predicts potential nerve supply to the joints of the shoulder by accompanying muscle innervation. The structures and potential nerves are summarized in Table 1.
Generally, degenerative disease of the shoulder includes: 1) cartilaginous injury, osteoarthritis, and inflammatory arthridities, 2) soft tissue disorders such as tendinitis, bursitis, tendinopathy, tendon tear, and adhesive capsulitis, 3) instability and impingement disorders. Advanced shoulder disease rarely manifests in a sole anatomic location and often presents with a constellation of joint and soft tissue findings. As in many other forms of chronic pain, central sensitization can play a role in the clinical course whether the cause is originally suspected to be peripheral or central (eg, post-stroke shoulder pain).[3-6] Nerve entrapment (eg, of the suprascapular nerve) is a known source of peripheral neuropathic pain.[7] Treatments for chronic shoulder pain are as diverse as the etiologies.[8-14] Exercise and physiotherapy are important foundations of treatment.[15-17] Besides NSAIDS and other forms of medical management,[9] steroid injections of the shoulder are a common treatment for musculoskeletal causes of shoulder pain. For adhesive capsulitis in particular, steroid injection may slightly improve range of motion outcome compared to conservative treatment.[10] Steroid injections are, however, largely a short- to intermediate-term treatment and probably do not affect final outcome for chronic pain over other forms of treatment.[9]
Treatments for chronic shoulder pain are as diverse as the etiologies.
Shoulder surgery may be required to address rotator cuff tears, and total shoulder joint replacement is a consideration for advanced and functionally limiting glenohumeral joint disease. However, patients may have contraindications to shoulder surgery such as medical comorbidities, neuropathic injury, or degree of rotator cuff tears.[18] While regenerative medicine has been proposed as a nonoperative option for treatment of shoulder pathology,[19] it has been most rigorously studied in the postoperative management of rotator cuff tear repair[20-24] as a way to reduce re-rupture. For nonoperative rotator cuff pathology, it has been studied with mixed outcomes and little research has been done to evaluate its efficacy in other shoulder pathologies.[21] Peripheral nerve stimulation has been reported for chronic shoulder pain treatment but presents with technical limitations for targeting all nerves innervating the shoulder joint.[24]
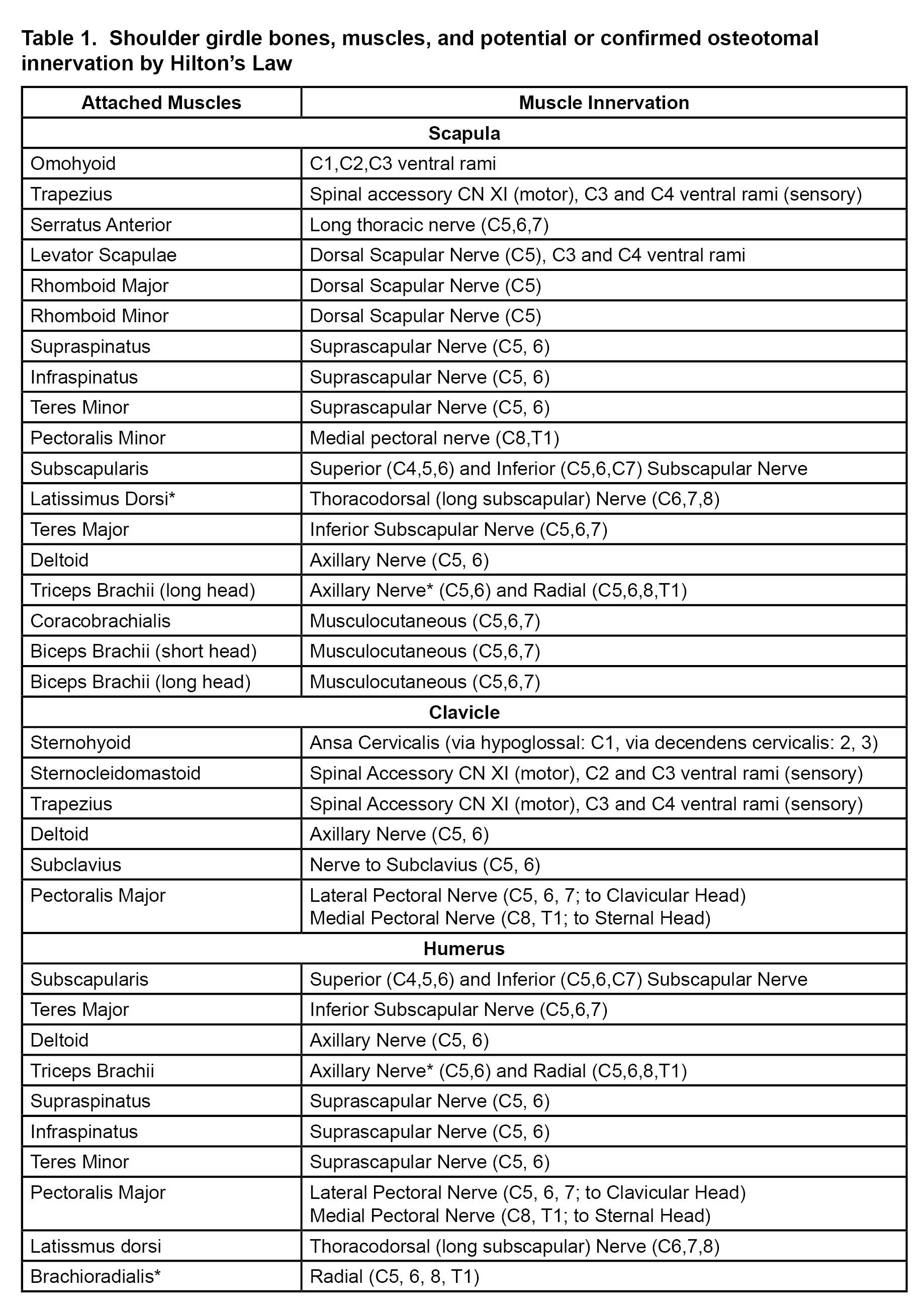
Both thermal and pulsed radiofrequency ablation (RFA) of the suprascapular nerve have been described to treat chronic shoulder pain,[14] but there is a theoretical concern of post-ablation weakness in the supraspinatus and infraspinatus muscles. Eckmann et al, have described the articular innervation of the glenohumeral joint for future techniques in joint denervation via nerve ablation.[25-28] Distal articular sensory denervation can reduce the chance of post-ablation motor weakness. Successful shoulder articular sensory denervation using RFA has been described in the literature.[29],[30] So far, the principal four nerves that innervate the glenohumeral joint and subdeltoid bursa are the suprascapular nerve, axillary nerve, nerve to subscapularis, and, occasionally, the lateral pectoral nerve. Of note, the lateral pectoral nerve provides innervation to the acromioclavicular joint and associated coracoacromial, coracoclavicular, and coracohumeral ligaments. The safe zones for ablation have been defined as the area lateral to the spinoglenoid notch posteriorly (suprascapular branches), at the inferior-posterior portion of the greater tubercle (axillary branches), and over the coracoid process (lateral pectoral branches).[25],[26] These safe zones are depicted in Figure 1. The lateral trunk of the suprascapular nerve also could be ablated (which may spare supraspinatus function but compromise infraspinatus function) midway between the suprascapular notch and spinoglenoid notch in the supraspinous fossa.[28] Nerve to subscapularis should be accessible over the anterior superior neck of the glenoid;[27] however, due to the proximity of this nerve to the brachial plexus and axillary artery, pre-clinical and clinical work on determining ideal ablation trajectory is ongoing by our team. As with the knee and hip joint, shoulder articular ablation is a compromise between full joint denervation and safety. Importantly, the inferior portion of the glenohumeral joint anteriorly and posteriorly is unacceptably close to the motor portion of the axillary nerve and the brachial plexus and too proximate to the circumflex humeral artery to be considered for ablation with perhaps the exception of rare palliative situations.
Herein we describe techniques to block and ablate articular sensory branches of the suprascapular, axillary, lateral pectoral nerves, and nerve to subscapularis using fluoroscopic guidance. We caution that these techniques are emerging and continue to require further pre-clinical and clinical study. We recommend that clinicians study the relevant anatomy closely prior to nerve ablation and disclose potential risks to patients in a fully transparent fashion.
Patient Selection
Based on aforementioned preclinical studies of articular nerves to the glenohumeral and/or acromioclavicular joints, ideal patients for articular sensory denervation should have clinical evidence of symptomatic osteoarthritis. Candidates may have chronic shoulder pain of suspected peripheral origin (ie, not of central origin) that has persisted despite rational multimodal therapy, especially if they are not good surgical candidates. Ablation of articular sensory nerves to the shoulder most likely has efficacy for pain originating from the joint capsule and nearby ligaments or from the nerves themselves. How denervation can diminish sensory input from rotator cuff injuries is unclear, though some patients with primarily rotator cuff disease did respond to ablation.[30] The role of ablation for treating chronic pain after total shoulder arthroplasty also requires further study, though some patients in this category did respond as well.[30]
Diagnostic blocks should be performed to identify patients who should proceed to have ablation. This is standard with other ablation techniques of the spine and major joints. A patient with greater than 50% pain relief is considered to have a positive diagnostic block.[30] Further studies are needed to determine the ideal number of blocks, block relief threshold, and volume of injectate for the diagnostic block. Inadvertent intra-articular injections are possible with this procedure. While intra-articular injections have diagnostic value, they probably have less prognostic value for RFA success; this phenomenon has been observed in other anatomic structures such as the lumbar zygapophyseal joint.[31] Nerve selection should follow zones of pain perception. Deep posterolateral pain would suggest that the suprascapular and axillary nerve should be targeted. Anterior pain may suggest that the lateral pectoral nerve and nerve to subscapularis should be targeted.
Safety considerations for both diagnostic blocks and RFA share commonalities with other pain interventions of low to intermediate risk. There is a low but possible risk of vascular, nerve/plexus injury, and joint infection. Extra caution must be taken for patients with implanted cardiac devices. These devices can be close to the site of nerve ablation (eg, lateral pectoral nerve ablation on the same side as the device). For this reason, we recommend the use of strict cardiac device protection protocols suitable for surgical electrocautery and in accordance with facility policies and procedures.
Diagnostic Block Technique: Prone Approach
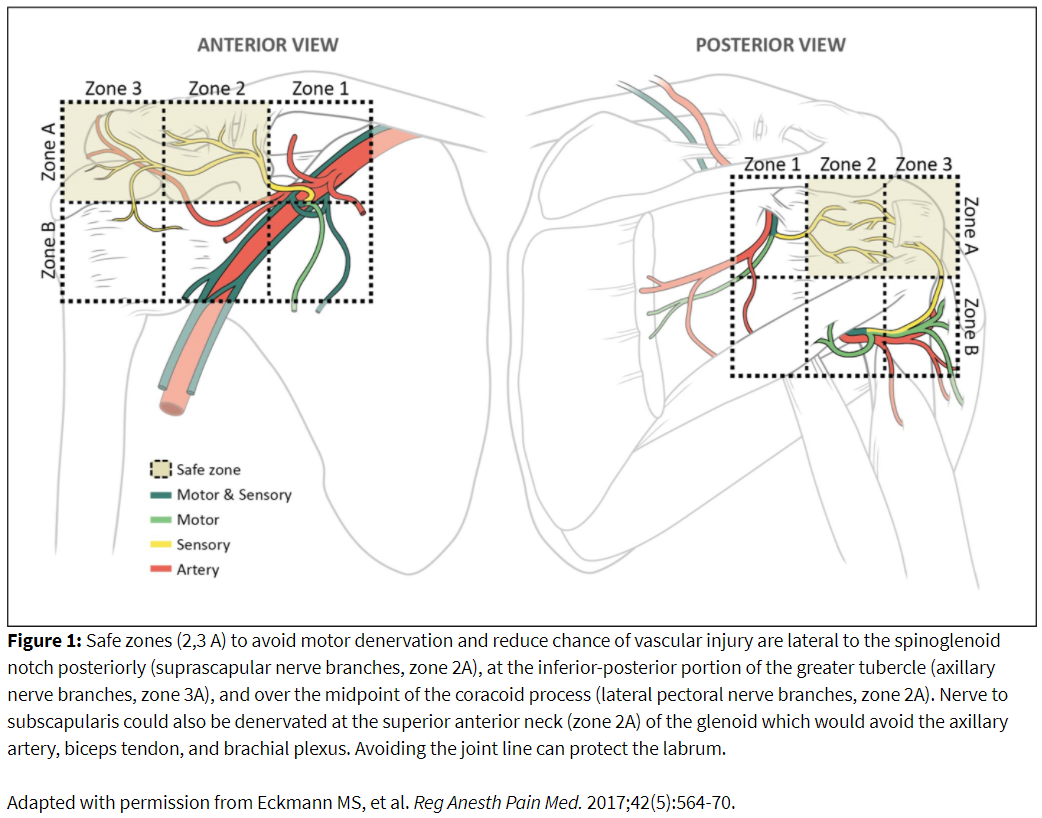
An annotated image, needle placement, and ablation zone is illustrated in Figure 2. Prone positioning provides access to the posterior shoulder joint for denervation of suprascapular and axillary articular branches. The patient is positioned with the operative arm at the side to provide optimal visualization of the humerus. The image is obtained with ipsilateral obliquity to obtain the Grashey or true anterior-posterior view of the glenohumeral joint. Additional modification of this view by declining the angle caudad provides better visualization of the glenoid neck and head of the humerus along with reducing image artifact created by the spine of the scapula. The spinoglenoid notch (ie, great scapular notch) can be identified by identifying the lateral border of the scapular spine to its attachment with the neck of the scapula.
The greater tubercle appears as a step-off on the lateral head of the humerus with a tapering at its inferior margin. If the head of the humerus appears rounded, this might indicate that the tubercle is anteriorly positioned, and further oblique rotation of the image is required. An ideal view of the glenoid neck and the greater tubercle can be achieved in the same orientation, requiring no further adjustment between the two targets.
Needle depth in adults can exceed 3 inches. The needle is advanced until contact with periosteum. Contrast confirmation is recommended to exclude intravascular or intraarticular uptake. Block volume should be approximately 0.5 ml of local anesthetic.
Diagnostic Block Technique: Supine Approach
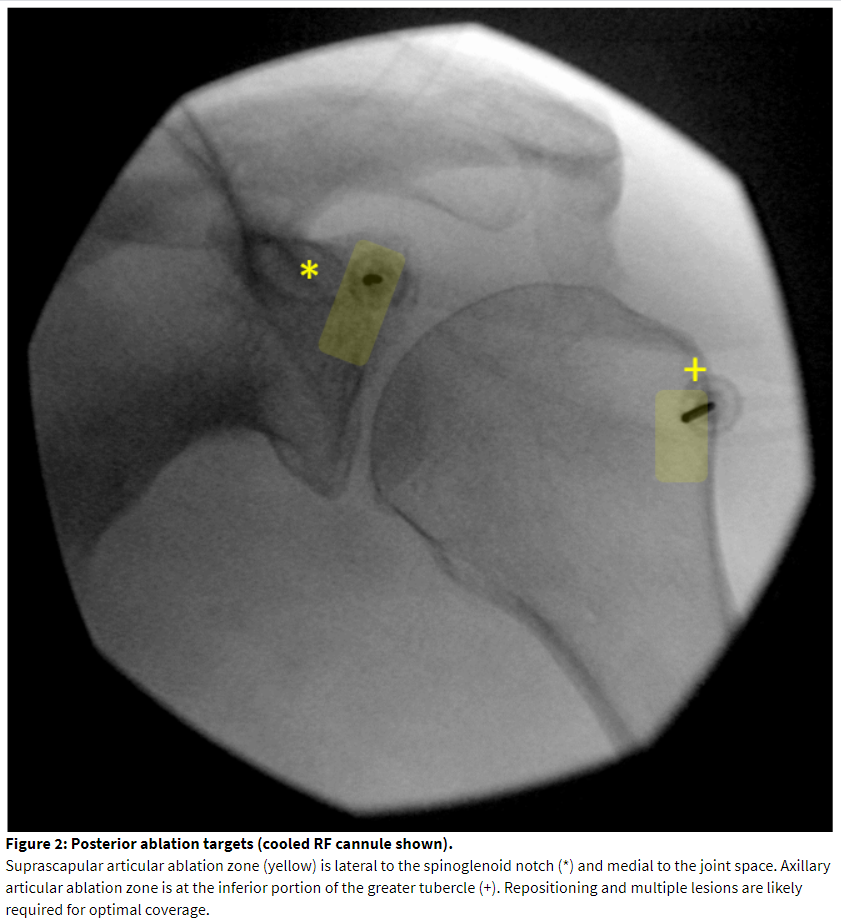
Supine positioning provides access to the lateral pectoral nerve and the nerve to subscapularis. An annotated image, needle placement, and ablation zone is illustrated in Figure 3. The patient is positioned with the operative arm at the side. The coracoid process should be visualized. In the neutral anteroposterior view, it will appear as a circle or oval image. Cephalad and ipsilateral rotation will elucidate better contour of the superficial surface of the coracoid process. This also will show the joint line and the neck of the glenoid. The superficial midpoint of the coracoid process is a recommended target for lateral pectoral block and ablation[29] and is often 2-3 cm below the skin surface. Placing the needle in the superior-anterior-lateral aspect of the neck of the glenoid will block the articular branch from the nerve to subscapularis.[27] This is approximately 1-1.5 cm deeper than the coracoid target. If both posterior and anterior targets are needed for the case, care must be taken to carefully assist the patient in flipping.
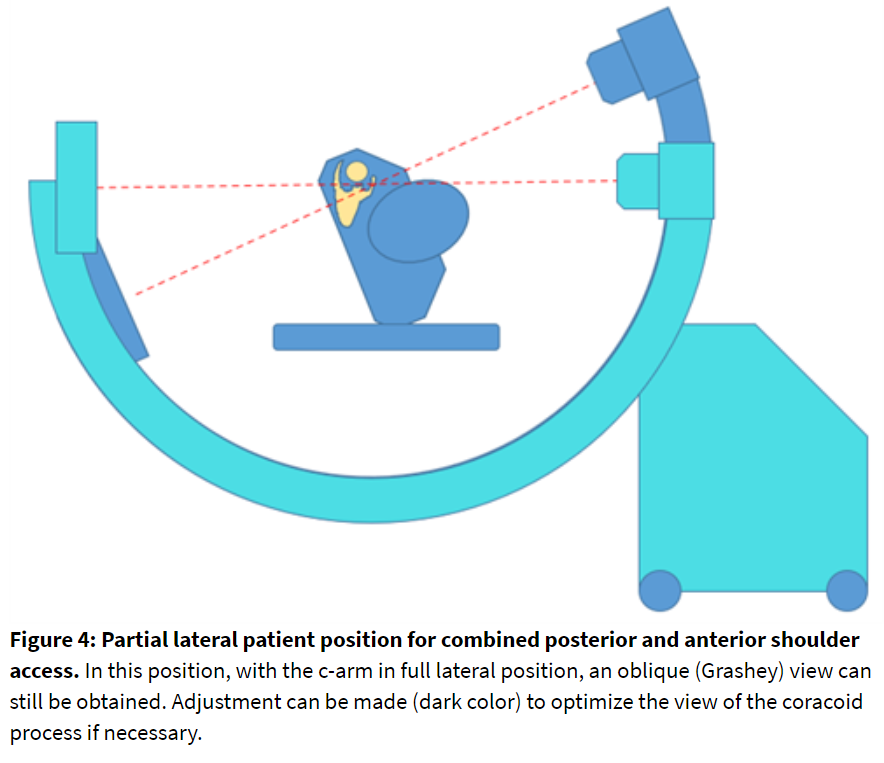
Alternate Position: Partial Lateral
Assuming both anterior and posterior nerves need to be blocked, the patient can be positioned in the lateral decubitus position. A partial lateral position (Figure 4) can provide proper joint visualization with fluoroscopy. The C-arm orientation should be evaluated in advance. Image acquisition and needle stabilization guidance can be more challenging in this position when compared to the staged prone-supine technique.
Access to the nerve targets is analogous to the described block technique. The planned ablation zone should replicate diagnostic block locations, hence the importance of limiting local anesthetic volume for diagnostic block. RFA can be painful, and sedation may be required. Sedation is not needed for diagnostic blocks and can confound analgesic interpretation of the procedure. Both standard thermal RFA and cooled RF thermal ablation have been performed.
Motor testing is recommended for shoulder articular ablation. Motor recruitment is a possibility. While patients with limited preexisting function may not be impacted by motor denervation, the goal of the procedure is to ablate predominantly sensory nerve fibers and preserve strength of the rotator cuff muscles.
Suprascapular articular ablation can be achieved in the top half of the posterior glenoid neck lateral to the spinoglenoid notch but medial to the joint line (Figure 2). Care should be taken not to enter the joint to reduce cartilaginous injury. The cannula will traverse the infraspinatus muscle, so care should be taken to minimize local trauma by minimizing the number of needle passes. Motor testing can be performed up to 1.5-2 volts (2 hertz). Absence of supraspinatus and infraspinatus muscle contractions should be confirmed. If there is a motor response, move the needle laterally while taking care not to traverse the labrum or joint space. Small twitches may be accepted at the discretion of the practitioner. The active tip may need to be repositioned to achieve coverage of the ablation zone. A total of 2-3 lesions can be performed in this area.
Axillary articular ablation can be performed inferior to the greater tubercle near the metaphyseal-diaphyseal junction of the humerus (Figure 2). The cannula tip will lay inferior to the distal teres minor tendon attachment. Several sensory fibers emanate from the mixed portion of the axillary nerve more medially on the posterior humeral head. Ablation in this area can increase the risk of motor denervation. Inferomedial placement close to the quadrangular space can cause trauma to the circumflex humeral artery. Absence of deltoid, teres minor, or long head of the triceps contraction should be confirmed. Positioning the active tip of the RF needle more laterally and superiorly on the head of the humerus will avoid motor recruitment. Two lesions in this area are recommended to maximize the ablation zone.
Lateral pectoral nerve ablation is performed over the mid-portion of the coracoid process from an anterior approach (Figure 3). This location is superficial. If the depth of the cannula tip is less than 1.5 cm, consider alternate methods for ablation such as chemical neurolysis in order to reduce the risk of skin injury. Placing a standard RF cannula in a medial to lateral and caudal to cranial direction matches the path of the nerve.[28] Ultrasound can be as a sole imaging modality. The accompanying branch of the thoracoacromial artery serves as a landmark for needle placement. If ultrasound is not used, we recommend placing a small gauge “finder” needle to exclude vascular return and protect against vascular trauma with a large bore RF cannula. Pectoral muscle stimulation should be excluded but is rarely encountered. If pectoral muscle contraction is encountered, the needle tip should be redirected laterally on the coracoid process. Contraction of the upper extremity should alert the practitioner to inadvertent positioning of the needle deep to the coracoid process and close to the brachial plexus.
Ablation of the nerve to subscapularis is undergoing preclinical and clinical investigation at the time of this publication. The authors have blocked the articular branch with success. The target can be seen in the same fluoroscopic view as the lateral pectoral block as described above. The needle tip should contact the upper lateral aspect of the anterior glenoid neck, traversing cranially to the coracoid process, from an anterior approach. The needle tip should remain medial to the joint line to avoid injury to the labrum and attachment of the biceps tendon. The needle tip should not be caudad to the coracoid process because this can endanger the brachial plexus and axillary artery. For this reason, the coracoid process is referred to as the “Lighthouse of the Shoulder.”
Post-Procedure Care
A motor examination of the shoulder should be performed. If a substantial amount of local anesthetic was required for RFA, a motor block can occur. If upper extremity weakness is present due to the effect of local anesthetic, an arm-sling should be provided to the patient. Patients and providers should be vigilant on the symptoms and signs of joint infection.
Passive range-of-motion exercises should be initiated after treatment. Patients should be referred to a qualified physical therapist for evaluation and training. Active range-of-motion exercise can be initiated as tolerated within 2-4 weeks following the procedure. Improved range of motion after shoulder nerve ablation has been demonstrated in the published literature.[14] Patients should inform their physician if there is a new character of pain or functional deficit. Patients should be educated that pain relief after RFA may take up to 2-4 weeks with a possibility of short-term increase in pain immediately after the RF procedure. Acute analgesic therapy may be required. Topical analgesic and hot or cold packs can be considered in addition to oral pain medications. The role of steroid injection during RFA is undetermined; in our practice, we do not routinely inject steroid during the ablation with the hypothetical goal of allowing secondary injury processes to occur in the thermal ablation zones.
Summary
Articular innervation of the glenohumeral joint, acromioclavicular joint, surrounding ligaments, and bursae have been described using quantitative anatomy techniques. Clinical success in the ablation of fibers from the suprascapular, axillary, and lateral pectoral nerves has been reported.[30] The nerve to subscapularis is a possible target for anterior shoulder pain. Ablation procedures of the shoulder should be performed with attention to anatomy, safety, and consideration of patient-centered risks and benefits in accordance with medical societal guidance. Additional research is warranted to improve patient selection, technique, pain relief, and functional outcomes.
References
- Kuijpers T, van Tulder M, van der Heijden G, Bouter L, van der Windt D. Costs of shoulder pain in primary care consulters: a prospective cohort study in The Netherlands. BMC Musculoskelet Disord. 2006;7:83. https://doi.org/10.1186/1471-2474-7-83
- Hébert-Blouin MN, Tubbs RS, Carmichael SW, Spinner RJ. Hilton's law revisited. Clin Anat.2014;27(4):548-55. https://doi.org/10.1002/ca.22348
- Noten S, Struyf F, Lluch E, D'Hoore M, Van Looveren E, Meeus M. Central pain processing in patients with shoulder pain: a review of the literature. Pain Pract.2017;17(2):267-80. https://doi.org/10.1111/papr.12502
- Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: central pain processing and shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13(1):81–9. https://doi.org/10.1016/j.jpain.2011.10.006
- Persson AL, Hansson GA, Kalliomäki J, Sjölund BH. Increases in local pressure pain thresholds after muscle exertion in women with chronic shoulder pain. Arch Phys Med Rehabil. 2003;84(10):1515–22. https://doi.org/10.1016/s0003-9993(03)00273-9
- Zeilig G, Rivel M, Weingarden H, Gaidoukov E, Defrin R. Hemiplegic shoulder pain: evidence of a neuropathic origin. Pain. 2013;154(2):263–71. https://doi.org/10.1016/j.pain.2012.10.026
- Labetowicz P, Synder M, Wojciechowski M, et al. Protective and predisposing morphological factors in suprascapular nerve entrapment syndrome: a fundamental review based on recent observations. Biomed Res Int. 2017;2017:4659761. https://doi.org/10.1155/2017/4659761
- Zheng XQ, Li K, Wei YD, Tie HT, Yi XY, Huang W. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(10):1824-31. https://doi.org/10.1016/j.apmr.2014.04.024
- Sun Y, Chen J, Li H, Jiang J, Chen S. Steroid injection and nonsteroidal anti-inflammatory agents for shoulder pain: a PRISMA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(50):e2216. https://doi.org/10.1097/MD.0000000000002216
- Sun Y, Lu S, Zhang P, Wang Z, Chen J. Steroid injection versus physiotherapy for patients with adhesive capsulitis of the shoulder: a PRIMSA systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore).2016;95(20):e3469. https://doi.org/10.1097/MD.0000000000003469
- Xiao RC, Walley KC, DeAngelis JP, Ramappa AJ. Corticosteroid injections for adhesive capsulitis: a review. Clin J Sport Med. 2017;27(3):308-20. https://doi.org/10.1097/JSM.0000000000000358
- Wu YT, Ho CW, Chen YL, Li TY, Lee KC, Chen LC. Ultrasound-guided pulsed radiofrequency stimulation of the suprascapular nerve for adhesive capsulitis: a prospective, randomized, controlled trial. Anesth Analg. 2014;119(3):686-92. https://doi.org/10.1213/ANE.0000000000000354
- Liu A, Zhang W, Sun M, Ma C, Yan S. Evidence-based status of pulsed radiofrequency treatment for patients with shoulder pain: a systematic review of randomized controlled trials. Pain Pract.2016;16(4):518-25. https://doi.org/10.1111/papr.12310
- Simopoulos TT, Nagda J, Aner MM. Percutaneous radiofrequency lesioning of the suprascapular nerve for the management of chronic shoulder pain: a case series. J Pain Res. 2012;5:91-7. https://doi.org/10.2147/JPR.S29864
- Wong CK, Levine WN, Deo K, et al. Natural history of frozen shoulder: fact or fiction? A systematic review. Physiotherapy. 2017;103(1):40-7. https://doi.org/10.1016/j.physio.2016.05.009
- Russell S, Jariwala A, Conlon R, Selfe J, Richards J, Walton M. A blinded, randomized, controlled trial assessing conservative management strategies for frozen shoulder. J Shoulder Elbow Surg.2014;23(4):500-7. https://doi.org/10.1016/j.jse.2013.12.026
- Kivimäki J, Pohjolainen T, Malmivaara A, et al. Manipulation under anesthesia with home exercises versus home exercises alone in the treatment of frozen shoulder: a randomized, controlled trial with 125 patients. J Shoulder Elbow Surg. 2007;16(6):722-6. https://doi.org/10.1016/j.jse.2007.02.125
- Drake GN, O'Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1526-33. https://doi.org/10.1007/s11999-009-1188-9
- Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13(1):1. https://doi.org/10.1186/s13018-017-0693-x
- Greenspoon JA, Moulton SG, Millett PJ, Petri M. The role of platelet rich plasma (PRP) and other biologics for rotator cuff repair. Open Orthop J. 2016;10:309-14. https://doi.org/10.2174/1874325001610010309
- Schneider A, Burr R, Garbis N, Salazar D. Platelet-rich plasma and the shoulder: clinical indications and outcomes. Curr Rev Musculoskelet Med.2018;11(4):593-7. https://doi.org/10.1007/s12178-018-9517-9
- Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41(10):2240-8. https://doi.org/10.1177/0363546513497925
- Ebert JR, Wang A, Smith A, et al. A midterm evaluation of postoperative platelet-rich plasma injections on arthroscopic supraspinatus repair: a randomized controlled trial. Am J Sports Med.2017;45(13):2965-74. https://doi.org/10.1177/0363546517719048
- Wilson RD, Bennett ME, Nguyen VQC, et al. Fully implantable peripheral nerve stimulation for hemiplegic shoulder pain: a multi-site case series with two-year follow-up. Neuromodulation. 2018;21(3):290-5.
- Eckmann MS, Bickelhaupt B, Fehl J, et al. Cadaveric study of the articular branches of the shoulder joint. Reg Anesth Pain Med. 2017;42(5):564-70. https://doi.org/10.1097/AAP.0000000000000652
- Bickelhaupt B, Eckmann MS, Brennick C, Rahimi OB. Quantitative analysis of the distal, lateral, and posterior articular branches of the axillary nerve to the shoulder: implications for intervention. Reg Anesth Pain Med. 2019;44:875-80. https://doi.org/10.1136/rapm-2019-100560
- Tran J, Peng PWH, Agur AMR. Anatomical study of the innervation of glenohumeral and acromioclavicular joint capsules: implications for image-guided intervention. Reg Anesth Pain Med.2019;44:452-8. https://doi.org/10.1136/rapm-2018-100152
- Tran J, Peng P, Agur A. Evaluation of suprascapular nerve radiofrequency ablation protocols: 3D cadaveric needle placement study. Reg Anesth Pain Med. 2019;44:1021-5. https://doi.org/10.1136/rapm-2019-100739
- Eckmann MS, Lai BK, Uribe MA 3rd, Patel S, Benfield JA. Thermal radiofrequency ablation of the articular branch of the lateral pectoral nerve: a case report and novel technique. A A Pract.2019;13(11):415-9.
- Eckmann MS, Johal J, Bickelhaupt B, et al. Terminal sensory articular nerve radiofrequency ablation for the treatment of chronic intractable shoulder pain: a novel technique and case series. Pain Med. 2020;21(4):868-71. https://doi.org/10.1093/pm/pnz335
- Cohen SP, Moon JY, Brummett CM, White RL, Larkin TM. Medial branch blocks or intra-articular injections as a prognostic tool before lumbar facet radiofrequency denervation: a multicenter, case-control study. Reg Anesth Pain Med. 2015;40(4):376-83. https://doi.org/10.1097/AAP.0000000000000229
Leave a commentOrder by
Newest on top Oldest on top