How I Do It: Sphenopalatine Ganglion Block and Radiofrequency Ablation
Cite as: Tolba R. How I do it: sphenopalatine ganglion block and radiofrequency ablation. ASRA News 2020;45. https://doi.org/10.52211/asra110120.072
Relevant Anatomy
The sphenopalatine ganglion (SPG), also known as the pterygopalatine ganglion or Meckel’s ganglion, is an extracranial parasympathetic ganglion that lies in an inverted pyramidal space called the pterygopalatine fossa. It is the largest ganglion outside of the calvarium containing sympathetic, parasympathetic, and sensory neurons.[1] Three main neural pathways intersect within the SPG. The sensory pathway accounts for a generous portion of the sensory innervation to the face and neck. The postganglionic sympathetic pathway arises from the superior cervical ganglion and courses through the calvarium as the deep petrosal nerve. The deep petrosal nerve combines with the parasympathetic greater petrosal nerve immediately before the foramen lacerum to form the nerve of the pterygoid canal, or Vidian’s Nerve. These sympathetic neurons coursing through the SPG provide vasoconstrictive innervation to the nasal cavity, upper pharynx, and palate.[2] The parasympathetic pathway originates in the superior salivatory nucleus of the pons. The preganglionic parasympathetic neurons course through the skull as the nervus intermedius, the geniculate nucleus, the greater petrosal nerve, and Vidian’s nerve before synapsing within the SPG. The postganglionic neurons provide secretomotor innervation to the lacrimal, nasal, palatine, and pharyngeal glands.[2]
The sphenopalatine ganglion is the largest ganglion outside of the calvarium containing sympathetic, parasympathetic, and sensory neurons.
Indications
The sphenopalatine ganglion block is employed for the treatment of sphenopalatine neuralgia, trigeminal neuralgia, atypical facial pain, acute migraine, cluster headaches, and herpes zoster.
Success with sphenopalatine ganglion blockade has been demonstrated in the treatment of chronic and episodic cluster headache. The mechanism of this success likely includes interruption of parasympathetic outflow from the superior salivary nucleus.
Clinical improvements have been demonstrated among patients with atypical facial pain, trigeminal neuralgia, and migraine headaches. In some cases, refractory pain has been improved in patients who have failed numerous noninvasive modalities.
Techniques for Sphenopalatine Block
Transnasal approach: The intranasal approach often is used as a diagnostic tool prior to percutaneous approaches or as an abortive treatment for acute symptoms. The technique utilizes a pledget soaked with local anesthetic that is advanced intranasally along the superior aspect of the middle turbinate overlying the SPG. There are also specialized local anesthetic delivery systems, which attempt to address anatomical limitations with flexible sheaths and angled catheters.[3,4] Advantages to the transnasal approach are its technical simplicity, short procedure times, and low overall risks. Risks are mostly limited to epistaxis and infection.[5, 6]
Transoral approach: Dentists are the primary users of this approach. The SPG is reached by a needle passing through the posterior end of the hard palate and the greater palatine foramen. This method can be technically challenging, and deposition of local anesthetic can be unpredictable.[5, 7]
Percutaneous infrazygomatic approach: Interventional pain physicians commonly use this approach, and it is the author’s preferred method. This technique is performed percutaneously under fluoroscopic guidance. It allows direct administration of local anesthetic to the SPG rather than diffusion across mucous membranes as seen in the intranasal approach. Fluoroscopic placement also allows subsequent radiofrequency ablation (RFA) of the SPG if temporary relief is achieved with the block.
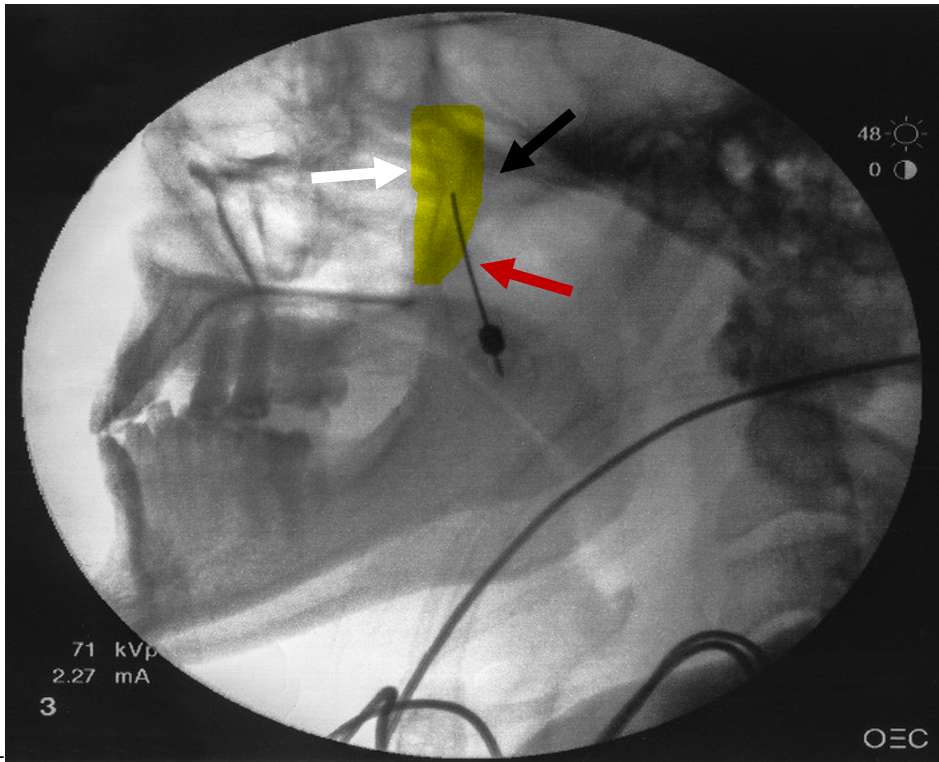
Figure 1: Lateral fluoroscopic view
White arrow indicates the pterygopalatine fossa that is highlighted in yellow. Black arrow represents the pterygopalatine plate located posterior to the fossa. Red arrow represents the needle with tip being advance superiorly and medially into the pterygopalatine fossa
Technique for Percutaneous Infrazygomatic Approach
- The patient is placed supine with their head in the neutral position. A lateral fluoroscopic view is obtained, and the head is adjusted to ensure the pterygopalatine fossa is well visualized. The mandibular rami should be superimposed.
- A skin wheal inferior to the zygomatic arch and anterior to the mandible is created with 1% lidocaine.
- A 22- or 25-gauge, 3½-inch spinal needle with a slightly bent tip is inserted coaxially with lateral fluoroscopic guidance. This view is the main view used while advancing the needle toward the pterygopalatine fossa. The needle is advanced superiorly and medially toward the pterygopalatine fossa (Figure1).
- An anteroposterior (AP) view is intermittently obtained to check the depth of the needle and avoid any breach to the nasal wall. The needle tip should terminate immediately lateral to the ipsilateral nasal wall as shown in the anteroposterior (AP view) (Figure 2).
- Following final needle positioning, 0.2 mL of contrast material is injected under live fluoroscopic imaging to rule out intravascular spread and confirm spread of the dye within the pterygopalatine fossa (Figure 3).
- Local anesthetic, such as 1-2 mL of 1% lidocaine with or without dexamethasone, is slowly injected into the fossa.
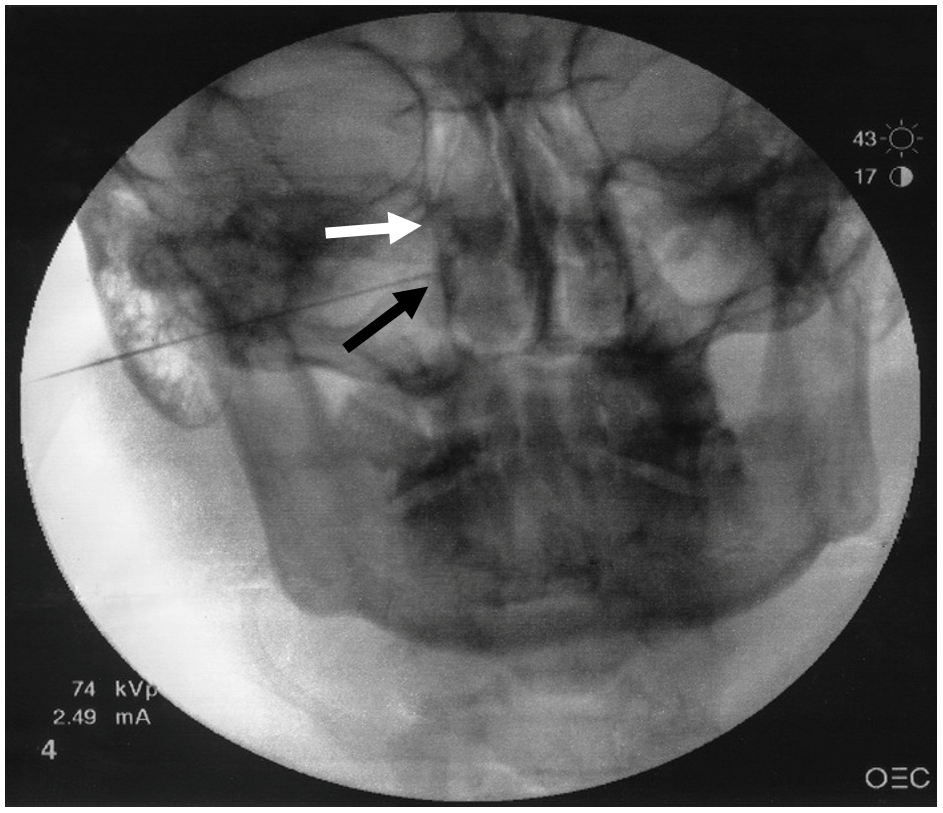
Figure 2. Anteroposterior fluoroscopic view.
White arrow represents lateral nasal wall. Black arrow represents needle tip lateral to ipsilateral nasal wall.
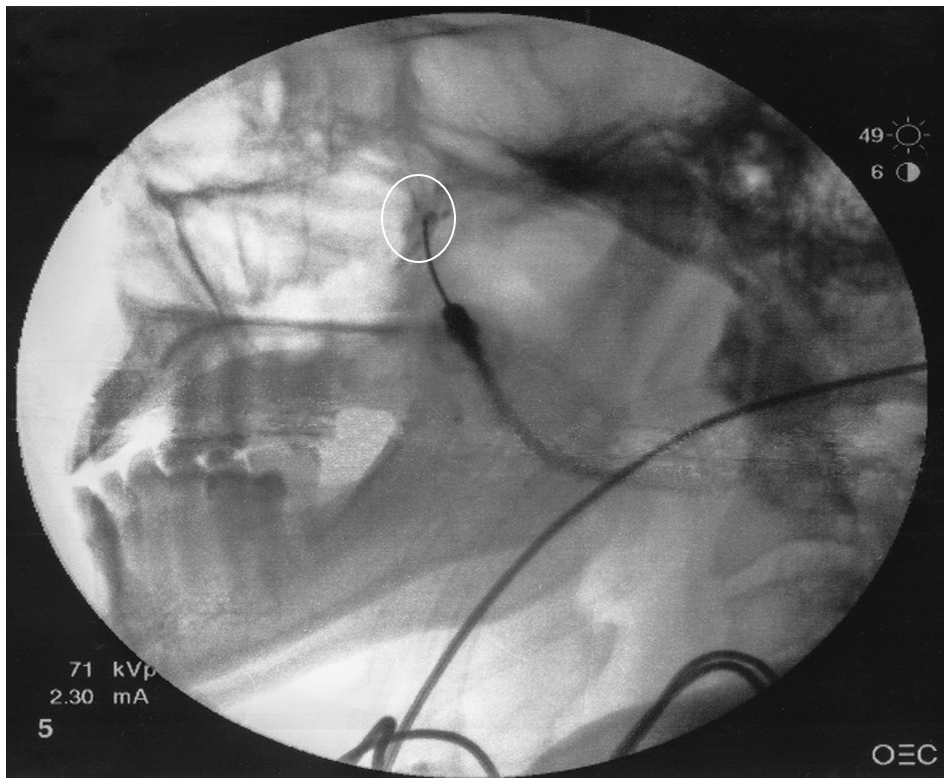
Figure 3. Lateral view with contrast dye
White circle represents dye spread in the pterygopalatine fossa
Radiofrequency Ablation of the SPG
For patients who responded favorably to SPG blocks, RFA can be performed. This can potentially provide longer-lasting pain relief for patients with conditions such as intractable cluster headaches.[6]
RFA of the SPG can result in temporary or, in rare cases, permanent hypoesthesia or dysesthesia of the palate, maxilla, or posterior pharynx. Reflex bradycardia has been reported during radiofrequency lesioning. This could be explained by the rich parasympathetic connections within the SPG.[8] Pulsed radiofrequency may be a safer method to avoid these effects; however, there are limited studies regarding its efficacy.[9,10]
Radiofrequency Ablation of the SPG – Technical Note
The infrazygomatic technique can be modified to perform RFA as follows:
- A 22-gauge, 10 cm, curved radiofrequency needle with a 5 mm active tip is advanced in the same manner as described above.
- On confirmation of proper needle position with negative intravascular spread, sensory stimulation is carried out at 50 Hz with a 1-millisecond pulse duration
- Deep perinasal and maxillary paresthesia at < 0.5 V signifies a favorable needle position.
- After stimulation and proper positioning are confirmed, local anesthetic is injected and neurotomy is performed at 80°C for 90 seconds with thermal conventional RFA. Pulsed RFA is an acceptable alternative to conventional thermal lesioning.
References
- ^ Lainez MJ, Puche M, Garcia A, Gascon F. Sphenopalatine ganglion stimulation for the treatment of cluster headache. Ther Adv Neurol Disord. 2014;7(3):162-8.
- ^ a b Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012;12(5):399-412.
- com: Dolor Technologies LLC; [cited 2018]. Available from: www.sphenocath.com. Accessed ??? 2020. [link doesn’t work]
- Cady R, Saper J, Dexter K, Manley HR. A double-blind, placebo-controlled study of repetitive transnasal sphenopalatine ganglion blockade with tx360® as acute treatment for chronic migraine. 2015;55(1):101-16.
- Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain Pract. 2012;12(5):399-412.
- ^ Narouze SN. Sphenopalatine ganglion block and radiofrequency ablation. In: Narouze SN Interventional Management of Head and Face Pain Nerve Blocks and Beyond. New York: Springer; 2014.
- Ruskin SL. Technique of sphenopalatine ganglion therapy for chorioretinitis. Eye Ear Nose Throat Mon. 1951;30(1):28-31.
- ^ Konen A.Unexpected effects due to radiofrequency thermocoagulation of the sphenopalatine ganglion: two case reports. Pain Dig. 2000;10:30-3.
- Bayer E, Racz GB, Miles D, Heavner J. Sphenopalatine ganglion pulsed radiofrequency treatment in 30 patients suffering from chronic face and head pain. Pain Pract. 2005;5(3):223-7.
- Narouze S. Complications of head and neck procedures. Tech Reg Anesth Pain Manag. 2007;11:171-7.
Leave a commentOrder by
Newest on top Oldest on top