A Comparison of Various Peripheral Nerve Stimulators Available Currently on the Market
Cite as: Singh V. A comparison of various peripheral nerve stimulators available currently on the market. ASRA Pain Medicine News 2022;47. https://doi.org/10.52211/asra110122.045
The field of peripheral stimulation has expanded in the past few years with multiple options available with external batteries, thus allowing interventional pain physicians to achieve peripheral stimulation with minimal incisions. Here we will review and compare these options in detail.
There are four different types of peripheral nerve stimulators with external pulse generators currently available: Sprint® (SPR Therapeutics), Stimrouter® (Bioventus), StimQ (Stimwave), and NaluTM. These are all approved by Food and Drug Administration (FDA) for chronic intractable pain of peripheral nerve origin. In addition, Sprint is approved for postsurgical and posttraumatic acute pain. It should be noted that the use of these peripheral nerve stimulators for the pain of cranial or facial nerve origin is not yet approved by the FDA.1 Table 1 shows the lead characteristics and program settings available for each, while Table 2 shows other unique characteristics. The Sprint system is unique as being an intentionally reversible, temporary 60-day implant.
Table 1: Lead characteristics and programming options
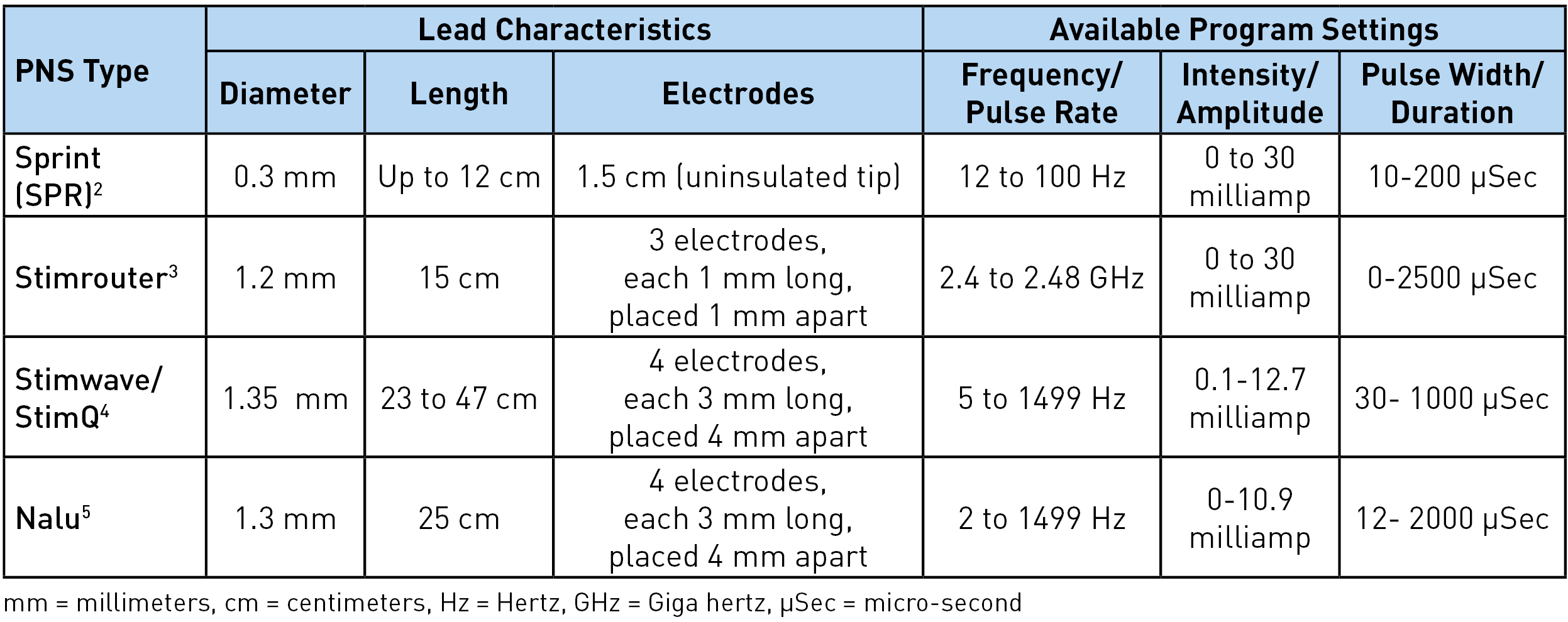
Table 2: Other unique characteristics
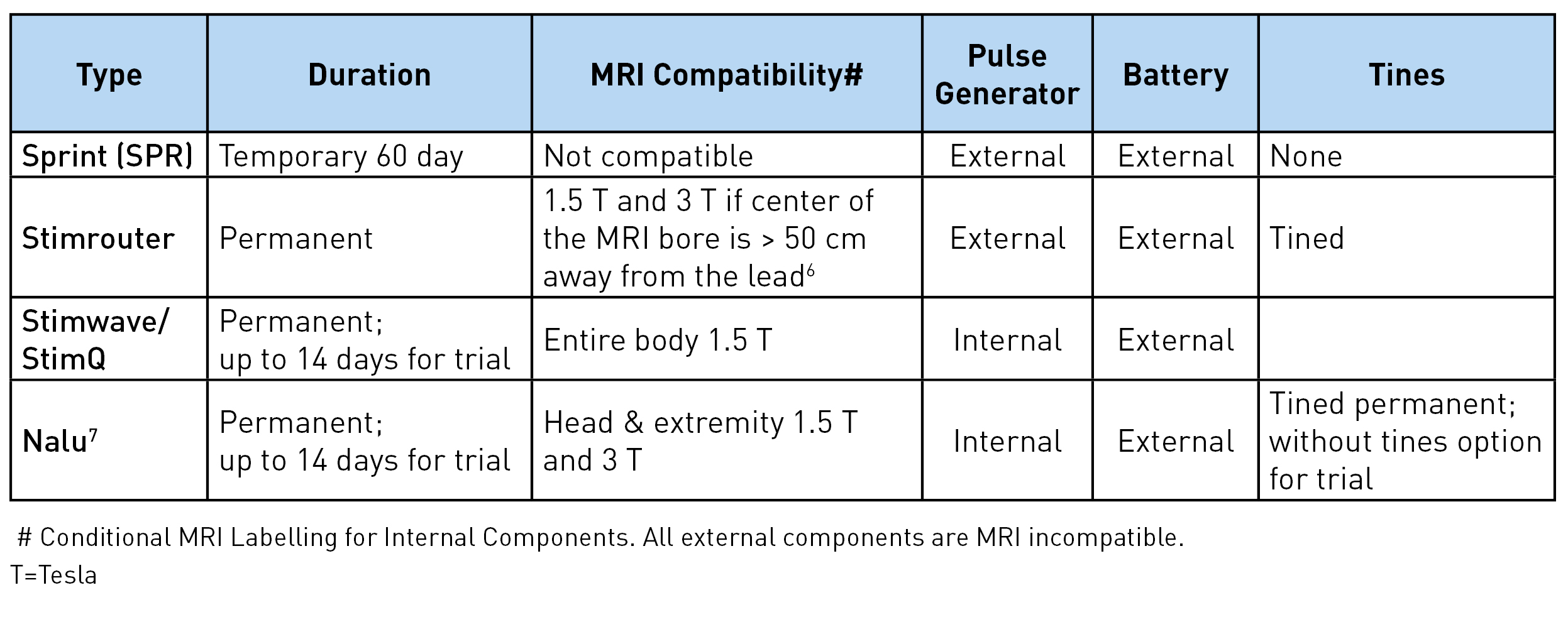
Sprint: The lead has a unique helical configuration (stretchable), reducing the risk of infection as it avoids the lead from going in and out of the skin. A waterproof bandage worn over the lead allows the patient to shower. However, swimming or bathing is not permitted. The external pulse generator and the battery are housed together and click on the mounting pad worn on the skin. The mounting pad serves a similar function as a grounding pad. The signal from the pulse generator is transferred to the electrodes via magnetic adapter cables. A minimal 2 cm of the lead should be implanted under the skin to reduce migration risk. Up to 12 cm (the length of the introducer lead) can be implanted, allowing great flexibility in terms of the lead insertion site. In usual clinical practice, 12 Hz frequency achieves muscle stimulation and 100 Hz achieves nerve stimulation. The entire system is magnetic resonance imaging (MRI) incompatible and has to be removed prior to MRI.
Stimrouter: The external pulse generator/transmitter and the battery are again housed together and snap on the conductive gel pad worn on the skin. This generates the stimulation signal and transmits the signal through the electrode to the lead. The electric arc is completed through the conductive gel pad. The receiver end of the lead must terminate in an area where the patient can tolerate surface stimulation and where the gel pad can adhere to the skin.3 For optimal stimulation, the ideal distance between the electrodes and the receiver end is 7.5 cm but up to 15 cm (assuming the entire lead is placed in one straight line) can work. Although the device can achieve up to 2.48 GHz, clinically, 2-200 Hz is used to avoid uncomfortable surface stimulation. The lead has multiple restrictions for MRI, including minimal distance from the center and the core of the MRI. Manufacturers' guidelines should be followed.8
Stimwave/StimQ: The pulse generator is located behind the electrode and is part of the lead itself. Any electrode can be positive or negative, allowing for the electric arc/circuit to complete at the level of the electrodes. This allows the higher frequency to be tolerated without any sensation at the skin level. Greater than 500 Hz in combination with low pulse width and amplitude achieves subthreshold in the periphery. The battery is worn externally over the distal end of the lead and communicates to the lead via a receiver radiofrequency wire inserted inside the electrode at the time of implant. Laying the lead on the body surface with the electrodes near the intended target can be helpful for planning the insertion site and tunneling the tail end. At least 23 cm of the lead has to be implanted, and the distal end (beyond the receiver marker band) can be cut. The internal lead is MRI compatible for the entire body at 1.5 T.9
Nalu: This lead and electrode configuration, as well as programming options, are very similar to the Stimwave. The biggest differences are in the pulse generator location, external battery configuration, and mode of energy transfer from the battery to the pulse generator. The pulse generator has a service life of 18 years.10 It is 2.8 cm long, 1.1 cm wide, and 4.9 mm thick and is connected to the lead and implanted in a small pocket created in the subcutaneous tissue. The battery is positioned externally over the pulse generator in an adhesive retention clip. The battery transfers energy to the generator via magnetic coupling, allowing the generator to send stimulation commands to the electrodes. The internal lead and implanted pulse generator are MRI conditional for head and extremity MRI if no part of the implanted system is within the transmit/receive radiofrequency coil of the MRI hardware component. Again, reading and following the manufacturer's guidelines prior to imaging is recommended.7
Patient selection for peripheral nerve stimulation has been discussed previously.11,12 Once a patient has been determined to be a candidate for peripheral nerve stimulation, the choice between the available systems should be determined based on
- Duration of the peripheral stimulation desired
- Need for trial
- Anatomy near the intended target
- The preferred approach for lead implant
- Patient's ability to place external battery as required, need for help with battery placement
- Need for future MRI
- Provider's comfort with the device and the peripheral nerve stimulator system
In conclusion, there are a variety of peripheral nerve stimulator options available in the interventional pain physician's armamentarium. Thoughtful selection of appropriate intervention is key to success.

Vinita Singh, MD, MS, is the director of cancer pain and an assistant professor in the department of anesthesiology at Emory University School of Medicine in Atlanta, GA.
References
- U.S. Food & Drug Administration. Commissioner O of the. U.S. Food and Drug Administration. FDA. Published August 10, 2022. Accessed August 11, 2022. https://www.fda.gov/home
- Sprint® PNS System. Accessed August 11, 2022. Available at: https://www.sprtherapeutics.com/sprint-pns-system/
- StimRouter. StimRouter® PNS resource center. Accessed August 11, 2022. https://stimrouter.com/physicians/stimrouter-pns-for-chronic-pain-management/resource-center
- Stimwave Technologies. Stimwave freedom stimulators StimQ PNS. Accessed August 11, 2022. Available at: https://stimwavefreedom.com/freedom-therapy/stimq-pns
- NaluTM Neurostimulation [product catalog]. Accessed August 11, 2022. Available at: https://nalumed.com/wp-content/uploads/2022/06/Nalu-Product-Catalog-MKT-400005-Rev-A.pdf
- Stimrouter Neuromodulation System. MRI guidelines. Accessed August 11, 2022. https://stimrouter.com/wp-content/uploads/2022/07/602-00730-001_Rev._A.pdf
- NaluTM Medical, Inc. NaluTM neurostimulation system surgical instructions for use: ported implantable pulse generator and leads. Accessed August 11, 2022. Available at: https://nalumed.com/wp-content/uploads/2022/07/Nalu-Ported-Implantable-Pulse-Generator-and-Leads-MA-000071-Rev-J.pdf
- Stimrouter. MRI safety. Accessed August 11, 2022. Available at: https://stimrouter.com/physicians/mri-safety-following-stimrouter-implant/
- Stimwave Technologies. MRI safety conditions for Stimwave stimulators. Accessed August 11, 2022. Available at: https://stimwavefreedom.com/about-us/mri-information
- Nalu. NaluTM neurostimulation system user instructions for use. Accessed August 11, 2022. Available at: https://nalumed.com/wp-content/uploads/2021/12/MA-000007-Rev-J.pdf
- Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;17(6):515-50. https://doi.org/10.1111/ner.12208
- Harrison NJ. Patient selection for peripheral nerve stimulation. The American Society of Regional Anesthesia and Pain Medicine (ASRA). Accessed August 29, 2022. Available at: https://www.asra.com/news-publications/asra-updates/blog-landing/legacy-b-blog-posts/2021/02/06/patient-selection-for-peripheral-nerve-stimulation
Leave a commentOrder by
Newest on top Oldest on top