POCUS Spotlight: Resuscitative Perioperative Transesophageal Echocardiography
Cite as: Perez d’Empaire P, Neethling E, Giron-Arango L, et al. POCUS spotlight: resuscitative perioperative transesophageal echocardiography. ASRA Pain Medicine News 2024;49. https://doi.org/10.52211/asra020124.014.
Transesophageal echocardiography (TEE) is an ideal intraoperative monitoring tool. It provides superior imaging when compared with transthoracic echocardiography (TTE), overcoming some of the challenges of TTE image acquisition, particularly in patients under mechanical ventilation or with large body habitus and in cases where the patient’s chest is part of the surgical field and not accessible. Although TEE is traditionally used in the cardiothoracic operating room, this review aims to describe the role of resuscitative perioperative TEE for non-cardiac anesthesiologists.
Basic TEE views
For this article, we will focus on four views. These views encompass the information needed to diagnose the etiology of perioperative hemodynamic instability. The examination is performed in two positions, the mid-esophageal (ME) level and the transgastric (TG) position (Figure 1). The manipulation of the probe towards the left and right, as well as adjustment of the multiplane angle, will allow the assessment of the regional walls and valves.
Used with permission: Carlos Guzman
Mid-Esophageal Four-Chamber (ME 4C) View
Obtaining the view: The ME 4C view is typically acquired at a depth of 30-34 cm. To ensure the proper depth is reached, consider the following:
- While advancing, from shallow to deeper areas, first locate the aortic valve, followed by the mitral valve, and finally the tricuspid valve until both ventricles are visible. If the ventricles are not clearly visible and seem small or distorted, it’s likely that you’re too shallow. Advancing the probe slightly will improve the view.
- Conversely, if the structures appear larger and seem to be filling more of the screen, or if you visualize structures typically seen at a greater depth as the coronary sinus positioned between the right atrium and right ventricle, you may be too deep and need to withdraw the probe slightly.
Multiplane angle: The standard angle for this view is typically set at 0 degrees. However, based on individual patient anatomy, slight adjustments might be necessary. The term “multiplane angle” pertains to the unique capability of the TEE probe. This probe is equipped with an array of piezoelectric crystals. When these crystals are electrically stimulated, they allow the direction of the ultrasound beam to be adjusted. This enables the beam to rotate in a clockwise direction from 0 to 180 degrees, all without the need for any physical movement of the probe itself.
Key features: In this view, clinicians get a comprehensive perspective, often described as a “bird’s eye view,” of the left ventricle (LV) and right ventricle (RV) as well as the mitral valve (MV) and tricuspid valve (TV). Diagnostic information that can be discerned from this view includes global RV and LV function and volume. The LV anterolateral and inferior septal regional walls can be assessed for regional wall motion abnormalities. This view is equivalent to the apical four-chamber view in TTE where both ventricles and atrium can be visualized for an overall assessment of cardiac function.
Assessment considerations: While more detailed assessments using color flow Doppler can identify valvular regurgitation or stenosis, for the purpose of the standard resuscitative echocardiography assessment, it’s advisable to keep things qualitative. This means focusing on visual observations and not diving deep into Doppler-based diagnostics unless necessary for the specific clinical scenario.
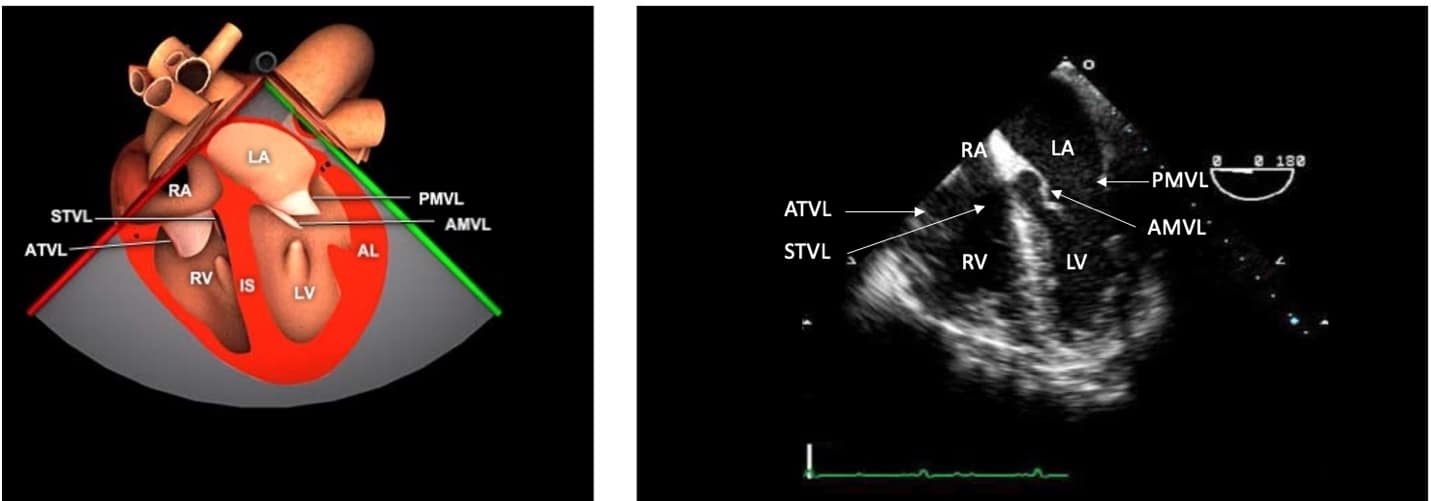
Figure 2. Mid-esophageal four-chamber (ME 4C) view.
ME = mid-esophageal, RA = right atrium, LA = left atrium, STVL = septal tricuspid valve leaflet, ATVL = anterior tricuspid valve leaflet, PMVL = posterior mitral valve leaflet, AMVL = anterior mitral valve leaflet, LV = left ventricle, RV = right ventricle.
Used with permission: https://pie.med.utoronto.ca/tee/
Mid-Esophageal Long Axis (ME LAX) View
Increase the multiplane angle from the ME 4C view to 120 to 130 degrees while keeping the probe centered over the MV. In this view, we can identify the left atrium (LA), anterior and posterior mitral valve leaflets (AMVL, PMVL), left ventricular outflow tract (LVOT), aortic valve (AV), and ascending aorta. This view allows for a more in-depth view of the ascending aorta. LV regional wall motion assessment can be performed for the inferior lateral and anterior septal walls.
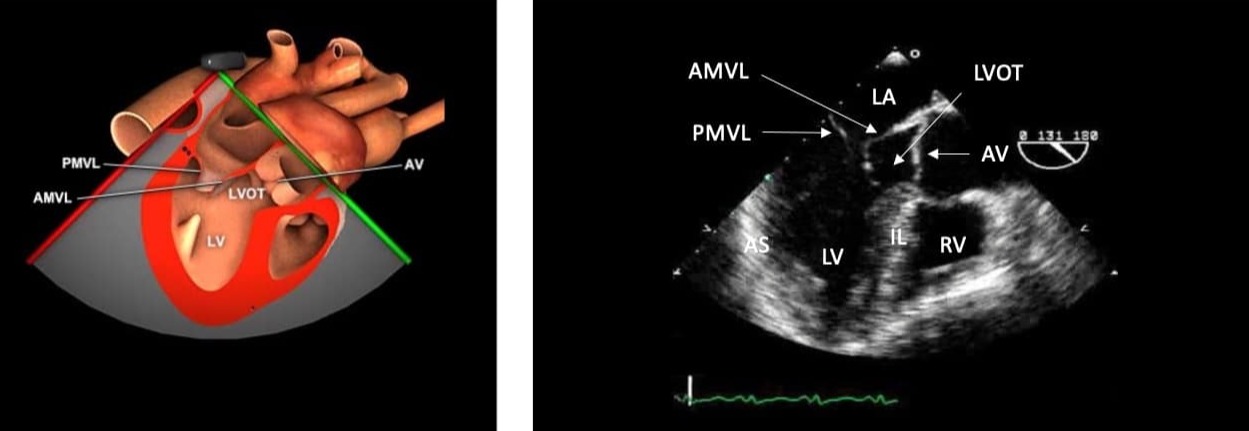
Figure 3. ME long axis view and anatomical correlation.
ME = mid-esophageal, LA = left atrium, AMVL = anterior mitral valve leaflet, PMVL = posterior mitral valve leaflet, IL = inferior lateral wall, AS = anterior septal wall, LV = left ventricle, RV = right ventricle, LVOT = left ventricular outflow
tract.
Used with permission: https://pie.med.utoronto.ca/tee/
Mid-esophageal Bi-Caval View
From the MELAX view, adjust the multiplane angle to 90 to 100 degrees. The LA, IAS, superior vena cava (SVC), inferior vena cava (IVC), RA, and right atrial appendage are visualized. One of the primary clinical utilities of this view for non-cardiac anesthetists, especially in emergencies, is the assessment of the IVC and SVC’s size and collapsibility. Evaluating the IVC provides valuable insights into a patient’s volume status. Additionally, this view allows for the evaluation of the IAS’s integrity, identifying potential septal defects or an open foramen ovale. Another important clinical utility is to guide and visualize the placement of central venous catheters, similarly the cannulation for extracorporeal life support, ensuring proper positioning and avoiding complications.
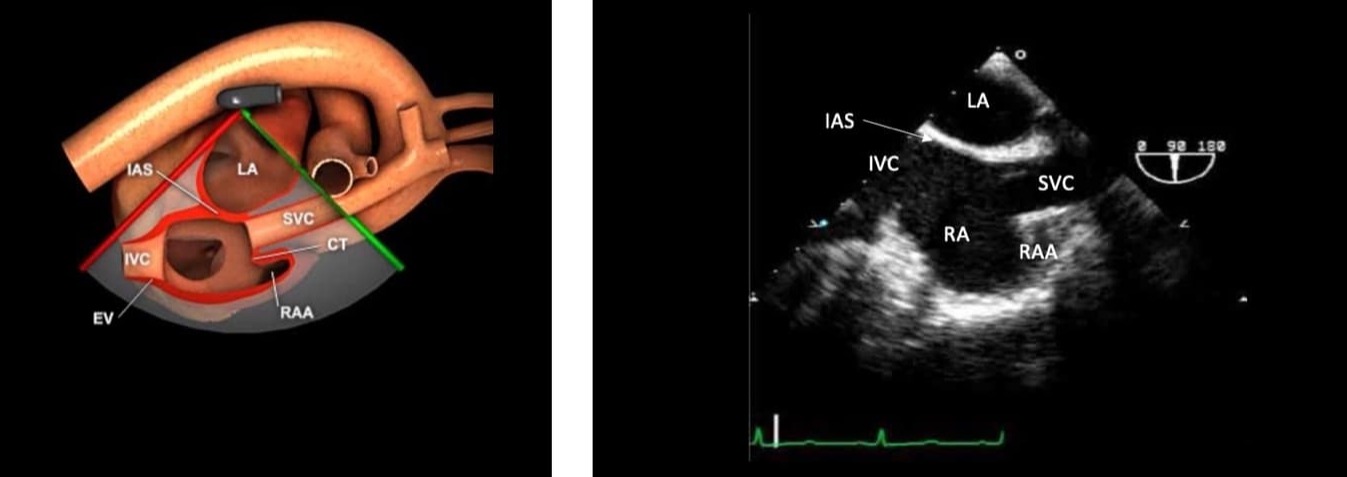
Figure 4. ME bicaval view.
ME = mid-esophageal, LA = left atrium, RA = right atrium, SVC = superior vena cava, IVC = inferior vena cava, IAS = intra atrial septum, RAA = right atrial appendage.
Used with permission: https://pie.med.utoronto.ca/tee/
Trans-Gastric Midpapillary Short Axis View (TG SAX)
This view is obtained by rotating the probe leftwards to center the image over the LV and then advancing the probe into the stomach. The LV can be assessed for size, function, volume status, and regional wall motion abnormalities. The intraventricular septal position aids in assessing ventricular interdependence.
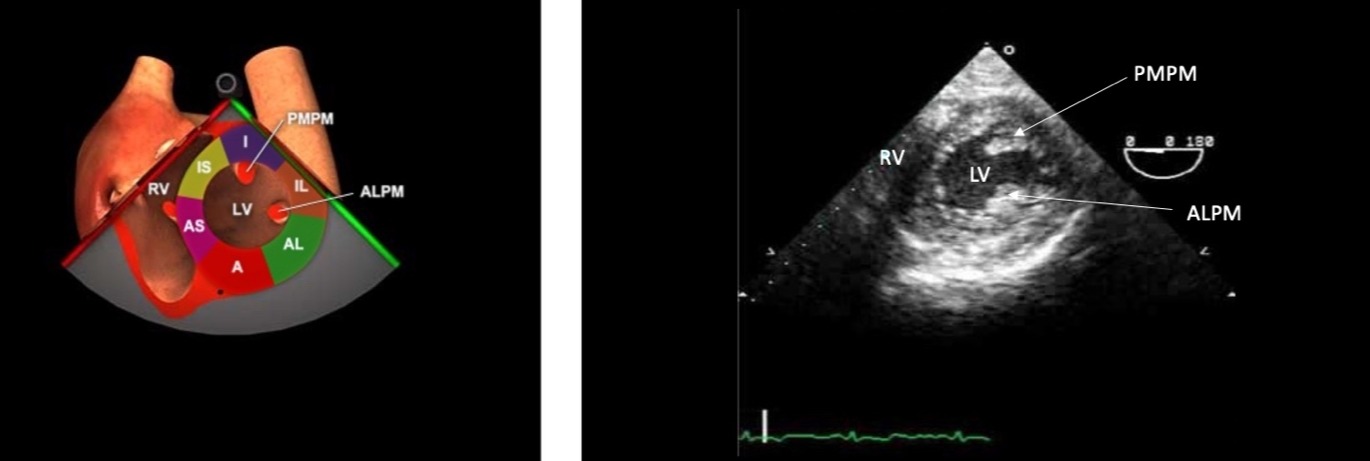
Figure 5. TG midpapillary SAX.
TG = Transgastric, RV = right ventricle, LV = left ventricle, PMPM = posterior medial papillary muscle, anterolateral papillary muscle, I = inferior, IL = inferolateral, A = anterior, AL = antero-lateral, AS = anterior septal, IS = inferior
septal.
Used with permission: https://pie.med.utoronto.ca/tee/
Clinical Applications of Resuscitative Perioperative TEE
Left and right ventricular function: Resuscitative TEE can serve as a valuable tool to qualitatively evaluate the global systolic function of both the LV and RV, especially in patients with previously unknown ventricular function.1
In Video 1, viewers can observe the heart in motion. Pay close attention to the myocardial thickening and the movement of the inner endocardium during the cardiac cycle. Normal LV function is evident when there’s noticeable thickening of the myocardium and adequate excursion (movement towards the center) of the inner endocardial lining during systole. These visual cues offer a qualitative (eye balling) assessment of LV function, helping clinicians gauge the heart’s pumping efficiency. Using the ME 4C and ME LAX views provides a comprehensive evaluation of global and regional LV function (Figure 6).2
The RV size can be qualitatively evaluated in relation to the LV. Typically, the surface area of the RV is approximately one-third or less of the LV’s surface area. Right ventricular hypertrophy and right atrial dilation indicate chronic RV pressure or volume overload. RV function can be quantified measuring the distance travelled by the tricuspid valve annulus in systole (TAPSE) using M-Mode in the ME 4C view. The normal TAPSE value is 17 mm (Figure 7).3
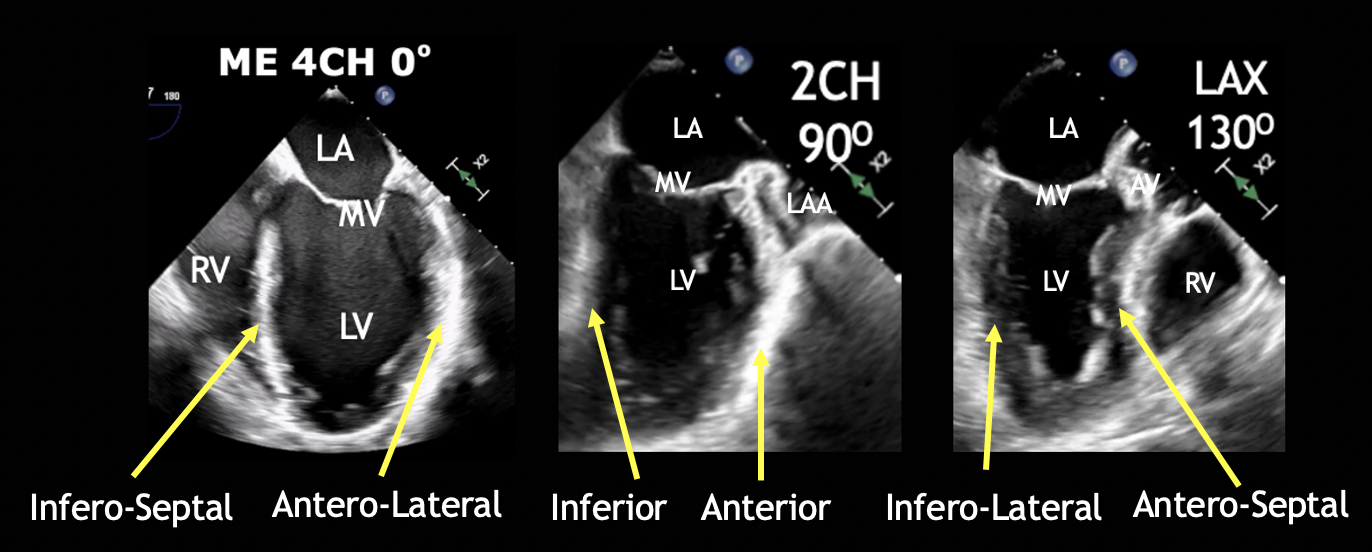
Figure 6. ME four-chamber, two-chamber, and LAX views and respective walls of the left ventricle.
CH = chamber, LA = left atrium, LAX = long axis, LV = left ventricle, RV = right ventricle, ME = mid esophageal, MV = mitral valve.
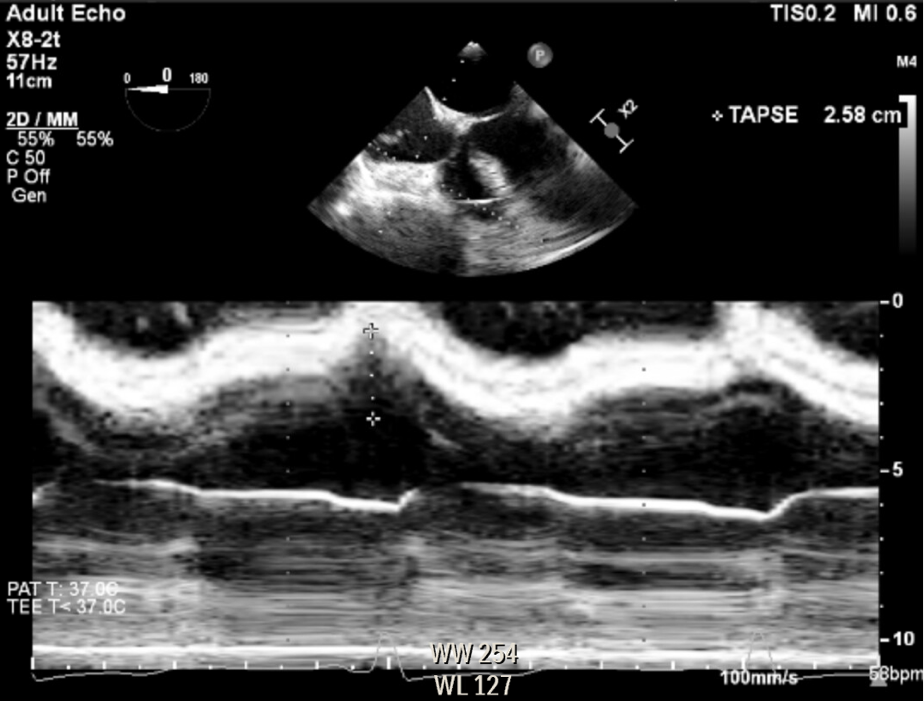
Figure 7. Right ventricle modified mid esophageal four-chamber view, measuring the distance traveled by the tricuspid valve annulus during systole (TAPSE), using anatomical M-Mode.
Left ventricular outflow tract obstruction: Dynamic left ventricular outflow tract (LVOT) obstruction is the third most frequent cause of unexplained hypotension.5 Systolic anterior motion (SAM) of the mitral valve is the most common cause of dynamic LVOT obstruction.6,7 SAM can lead to mal coaptation of the mitral leaflets, resulting in significant mitral regurgitation, further impairing cardiac output (Video 2).7 When LVOT obstruction is the cause of hypotension and low cardiac output syndrome, it will not respond to standard treatment, or it will even exacerbate following the administration of positive inotropic agents and vasodilators.7 LVOT obstruction can be identified using the ME five-chamber view, which is obtained by pulling the probe one or two centimetres from the ME 4C or by the ME LAX view. SAM is detected by observing the anterior mitral valve leaflet pulled towards the inter-ventricular septum through systole. Colour Doppler will show a mosaic pattern in the areas with turbulent flow due to aliasing (Video 2). The ME LAX view in TEE offers excellent visualization of this clinical problem during the operative period when access to the TTE apical four-chamber view is commonly limited by the surgical field. In addition having the TEE probe in place allows for continuous monitoring to guide clinical response to the medical interventions available to manage dynamic LVOT obstruction.
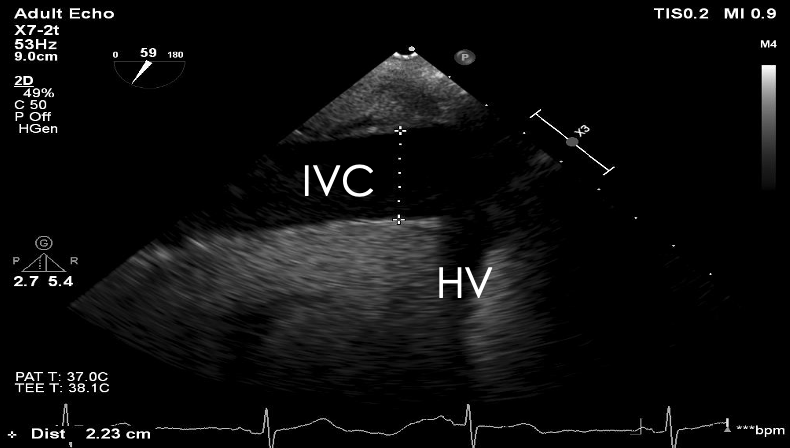
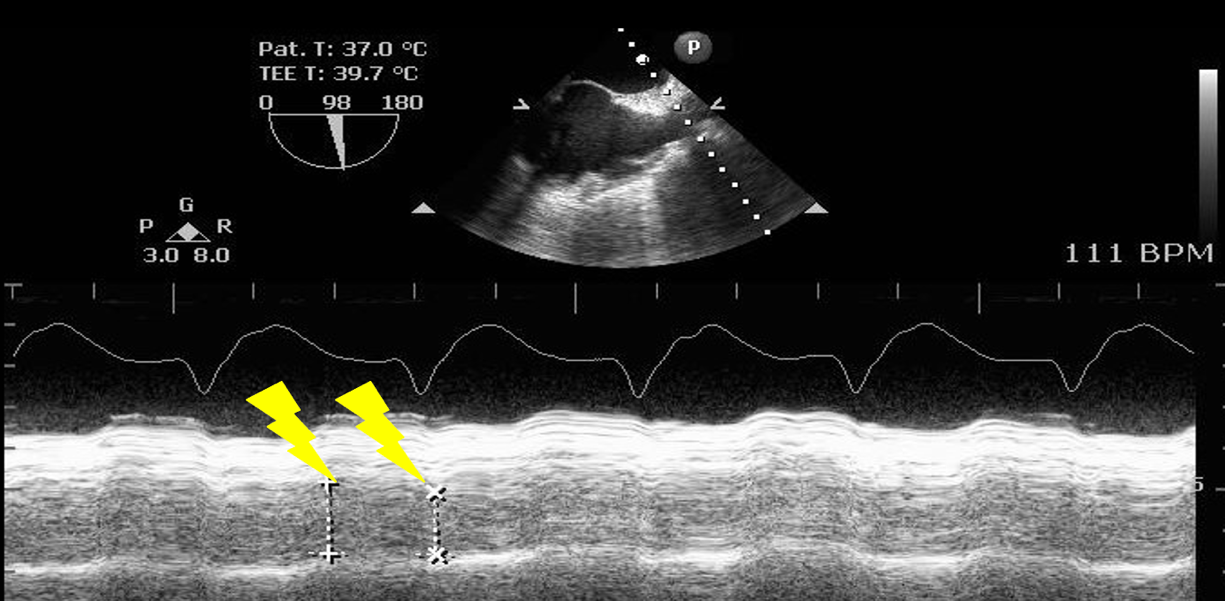
Hypovolemia: Volume status can be evaluated by directly assessing the right and left chambers and vessel size or by dynamic evaluation of blood flow. Using the TG SAX view, the left ventricle end-diastolic area (LVEDA) is the single recommended measurement to assess volume responsiveness (Video 3).1 A LVEDA < 8 cm2 or left ventricular fractional area change < 50% is a hypovolemic state.8-10 Using the ME bicaval view, the size and collapsibility of the IVC can also estimate the central venous pressure (CVP).11 The IVC should be measured 1 cm away from the hepatic vein origin; normal size is less than 2.1 cm with > 2.5 cm being severely dilated (Figure 8).12 Respiratory variation of the SVC (distensibility >36%), accurately predicts fluid responsiveness in mechanically ventilated patients (Figure 9).13
Cardiac tamponade: TEE has a diagnostic accuracy of nearly 100% for cardiac tamponade17,19 echocardiographic signs of tamponade include reduced left ventricular size leading to reduced stroke volume and dilated IVC.17 The absence of any cardiac chamber collapse has a >90% negative predictive value for clinical cardiac tamponade.20 As an early sign of tamponade, a systolic collapse of the right atrium is seen in the ME 4C view. When this collapse lasts for more than one-third of systole, it is a sensitive and specific indicator of cardiac tamponade.21,22 more specific but less sensitive is the early diastolic collapse of the right ventricle in the ME 4C. Finally, the left ventricle appears to be reduced in size with reduced end-diastolic and end-systolic dimensions and the appearance of left ventricular hypertrophy with reduced stroke volume and cardiac output (Video 4).17,20 IVC plethora, a sensitive sign occurring in >90% of cardiac tamponade cases, is a dilated IVC (>2.1 cm) with less than 50% reduction in diameter with spontaneous inspiration.17,20
Major valvular disease: Resuscitative TEE can be employed as an introductory tool for a qualitative examination of the heart’s valves, offering a preliminary insight into potential issues like valvular regurgitation and stenosis,1,23 such as:
- Mitral regurgitation (Video 5). Look for the presence of a regurgitant jet in the left atrium during systole. A prominent, bright-coloured jet that fills a substantial portion of the left atrium suggests a significant regurgitation.
- Aortic stenosis: Pay attention to the aortic valve’s opening during systole. A valve that doesn’t open fully or appears thickened and calcified might indicate stenosis. Secondary signs include hypertrophy of the left ventricle, which occurs in response to the increased pressure needed to pump blood through a narrowed valve.
These are basic assessments that give an introductory understanding. More nuanced assessments, such as evaluating eccentric jets or prosthetic valves, are better suited for an advanced TEE examination.1,2
Cardiac arrest: Utilizing TEE during cardiac arrest situations can offer several key insights: 22-24
- Optimization of chest compressions. TEE using the MELAX view can highlight the area of maximal compression, allowing for real-time adjustments to enhance the effectiveness of chest compressions. TEE can be done continuously during resuscitation, avoiding CPR interruptions or delays.
- Rhythm characterization. TEE aids in distinguishing the actual mechanical activity of the heart from the electrical rhythm. For instance, even in the presence of a non-shockable electrical rhythm, TEE can reveal pulseless ventricular fibrillation, emphasizing the importance of defibrillation without delay (Video 6). Essentially, it is crucial to discern if there’s an actual mechanical contraction of the heart that matches the detected electrical activity. A situation where there’s electrical activity without corresponding mechanical contraction is a clinical scenario known as “electromechanical dissociation.”
- Identifying reversible causes. TEE can help pinpoint reversible etiologies or triggers of cardiac arrest, guiding the management strategy.
- Procedural guidance. TEE can ensure proper placement of guide wires during central venous line insertion, assist in the insertion of an intra-aortic balloon pump, guide temporary transvenous pacemaker procedures, and ensure correct positioning of cannulas for extracorporeal circulation.
Limitations and Contraindications of TEE
To perform TEE safely, the patient needs to be sedated or intubated, and in case of sedation, fasting should be ensured. The distal portion of the ascending aorta is technically challenging to visualize due to the presence of air in the trachea; hence, a lesion on this portion can be missed. Contraindications that need to be taken into consideration before performing a TEE are summarized in Table 1.
Table 1: Absolute and relative contraindications of transesophageal echocardiography.
| ABSOLUTE CONTRAINDICATIONS | RELATIVE CONTRAINDICATIONS |
| Unclear or unstable cervical spine | Severe coagulopathy, thrombocytopenia |
| Esophageal pathology: severe dysphagia, stricture, acute and symptomatic esophagitis, esophageal tumor, diverticulum | Hiatal hernia (moderate to large) |
| Recent upper gastrointestinal surgery (< 6 weeks)* | Recent upper gastrointestinal surgery (6 weeks to 6 months) |
| Previous esophageal surgeries: esophagectomy, esophageal stent** | Recent gastric bleed (1-3 months, consider avoiding gastric views) and esophageal bleed (< 1 month) |
| Suspected unrepaired esophageal or gastric injury | Neck radiation < 3 months |
| Patient refusal | Esophageal varices with recent band ligation (7 days) caution with varices over 5mm |
| Esophagitis/peptic ulcer < 3 months with symptoms, history of Barrett’s with dysphagia |
*Needs discussion with surgery to perform.
**For surgeries with esophageal patency, TEE could be performed if done after more than 6 months.
It is advised that TEE could be performed in patients with oral, esophageal, or gastric disease if the expected benefits outweigh the potential risk. In patients with these pathologies, it is advised that to avoid unnecessary probe manipulation, the most experienced operator should be the one performing the TEE.
In summary, resuscitative TEE is an excellent point of care ultrasound modality to be used by anesthesiologists managing critically ill patients during the perioperative period. As a diagnostic tool, it can guide medical decisions during perioperative emergencies, such as shock or severe hypotension refractory to usual treatment, hypoxemia from unknown cause, and optimization of resuscitation in the case of cardiac arrest.




References
- Reeves ST, Finley AC, Skubas NJ, et al. Special article: basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg 2013;117(3):543-58. https://doi.org/10.1213/ANE.0b013e3182a00616
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26(9):921-64. https://doi.org/10.1016/j.echo.2013.07.009
- Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685-713; quiz 786-8. https://doi.org/10.1016/j.echo.2010.05.010
- Sherrid MV. Pathophysiology and treatment of hypertrophic cardiomyopathy. Prog Cardiovasc Dis2006;49(2):123-51. https://doi.org/10.1016/j.pcad.2006.08.001
- Ibrahim M, Rao C, Ashrafian H, et al. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg 2012;41(6):1260-70. https://doi.org/10.1093/ejcts/ezr232
- Sobczyk D. Dynamic left ventricular outflow tract obstruction: underestimated cause of hypotension and hemodynamic instability. J Ultrason 2014;14(59):421-7. https://doi.org/10.15557/JoU.2014.0044
- Bolliger D, Poltera C, Cheung AT, et al. Assessment of left ventricular dimensions by transoesophageal echocardiography in patients during coronary artery bypass surgery. Turk J Anaesthesiol Reanim 2017;45(6):367-73. https://doi.org/10.5152/TJAR.2017.25483
- Kou S, Caballero L, Dulgheru R, et al. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014;15(6):680-90. https://doi.org/10.1093/ehjci/jet284
- Porter TR, Shillcutt SK, Adams MS, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2015;28(1):40-56. https://doi.org/10.1016/j.echo.2014.09.009
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233-70. https://doi.org/10.1093/ehjci/jev014
- Vieillard-Baron A, Chergui K, Rabiller A, et al. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004;30(9):1734-9. https://doi.org/10.1007/s00134-004-2361-y
- Feissel M, Michard F, Mangin I, et al. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001;119(3):867-73.
- Slama M, Maizel J. Pulse pressure variations in acute respiratory distress syndrome: “fifty shades of grey.” Crit Care Med 2016;44(2):452-3. https://doi.org/10.1097/CCM.0000000000001529
- Mahjoub Y, Pila C, Friggeri A, et al. Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med 2009;37(9):2570-5. https://doi.org/10.1097/CCM.0b013e3181a380a3
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26(9):965-1012.e15. https://doi.org/10.1016/j.echo.2013.06.023
- Haaz WS, Mintz GS, Kotler MN, et al. Two dimensional echocardiographic recognition of the descending thoracic aorta: value in differentiating pericardial from pleural effusions. Am J Cardiol1980;46(5):739-43. https://doi.org/10.1016/0002-9149(80)90423-3
- Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36(42):2921-64. https://doi.org/10.1093/eurheartj/ehv318
- Spodick DH. Acute cardiac tamponade. N Engl J Med 2003;349(7):684-90. https://doi.org/10.1056/NEJMra022643
- Gillam LD, Guyer DE, Gibson TC, et al. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation 1983;68(2):294-301. https://doi.org/10.1161/01.cir.68.2.294
- Himelman RB, Kircher B, Rockey DC, et al. Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade. J Am Coll Cardiol1988;12(6):1470-7. https://doi.org/10.1016/s0735-1097(88)80011-1
- Meineri M. Transesophageal echocardiography: what the anesthesiologist has to know. Minerva Anestesiol 2016;82(8):895-907.
- Beaulac GR, Teran F, Lecluyse V, et al. Transesophageal echocardiography in cardiac arrest: the heart and beyond. Can J Cardiol 2023; 39(4):458-73. https://doi.org/10.1016/j.cjca.2022.12.027
- Teran F, Dean AJ, Centeno C, et al. Evaluation of out-of-hospital cardiac arrest using transesophageal echocardiography in the emergency department. Resuscitation 2019;137:140-7. https://doi.org/10.1016/j.resuscitation.2019.02.013
- Fair J, Tonna J, Ockerse P, et al. Emergency physician-performed transesophageal echocardiography for extracorporeal life support vascular cannula placement. Am J Emerg Med2016;34(8):1637-9. https://doi.org/10.1016/j.ajem.2016.06.038