Nerve Stimulator in Regional Anesthesia: Is it Out of Vogue?
Cite as: Khurana J, Ip VHY, Vijayashankar RS, Tsui B. Nerve stimulator in regional anesthesia: is it out of vogue? ASRA News 2020;45. https://doi.org/10.52211/asra110120.067
Introduction
Since the late 18th century, many pioneers have advanced our understanding of the electrophysiologic properties of nerve conduction.[1-3] Such groundbreaking works would eventually influence the creation and application of a nerve stimulator that is more portable and similar to those commonly used today.[4] Although this popular technology continued to undergo slight modifications over several decades, it was eventually “replaced” by the ultrasound (US) for guiding peripheral nerve blocks (PNBs).[5] Beginning in the late-1990s, the perceived advantages of such image guidance, including increased efficacy and safety, has earned US imaging the reputation as the absolute monitor for regional anesthesia.[5-7] Are these seemingly positive outcomes, however, potentially blinding us from improving patient care? Are too many operators depending solely on US for PNBs? We suggest that, in further perfecting our regional techniques, we must reflect on and retain some influences from our past.
Together, ultrasound and electronic nerve stimulation are complementary to each other and increase the likelihood that intraneural injections will be detected and prevented.
Although the prerequisite knowledge of surface landmarks/anatomy has remained constant, the technique of eliciting a paresthesia has been found to be potentially unreliable and possibly harmful as a needle is “blindly” advanced toward the target nerve.[5,8-11] Eliciting such abnormal stimuli was eventually supplemented with electrical nerve stimulation (ENS), which allowed performance of more difficult nerve blocks while decreasing reliance on the patient’s verbal feedback, reducing effective local anesthetic (LA) dose with probable reductions in morbidity.[4,12,13] The use of this technology allowed regional anesthesia to evolve rapidly over just a few decades. Briefly, ENS works by initiating ion flow in nearby neuronal cells causing either muscle contraction or paresthesia depending on nerve type (ie, motor/mixed vs sensory, respectively). When nerve stimulation is used to localize a nerve, a higher stimulating current is typically used, for example, 1-1.5 mA, and, as the needle approaches the target nerve, the current is reduced to a minimum until the lowest twitch response is seen or paresthesia is felt. After aspirating to rule out intravascular injection, a LA or normal saline (NS) test dose is often given, and a diminishing muscle response is noted (Raj Test).[14] The full dose of LA can then be injected. It was initially hypothesized that the small volume of LA or NS abolishes the motor response elicited by the low current via the injectate pushing the target nerve away from the needle. It was later proven, however, that both of these solutions are conducting fluids, and when a non-conducting fluid, such as dextrose 5% in water (D5W) is used, the elicited motor response is continued or augmented. This response occurs because D5W provides a uniform electric field around the needle tip rather than along the length of the needle like the aforementioned solutions.[15,16]
Despite its popularity for performing PNBs, ENS was eventually displaced by US as imaging technology began to increase in popularity and availability.[4,5,7] Many advocates for US-guidance cite its ability to reduce procedural time, improve block quality, and increase safety.[5,7] Various studies, however, have demonstrated that visually interpreting the proximity between the needle and nerve may not be reliable. For instance, Retter et al. performed US-guided supraclavicular blocks on cadavers and histologically found a 24% incidence of sub-perineural injections. While such a high incidence of neurological complications or cadaveric structural integrity are not seen in clinical practice, their findings demonstrate that the lateral resolution of the US is not high enough to differentiate intra- and extrafascicular injections.[17] Furthermore, although subclinical, one cannot advocate for intraneural injection since LA deposition directly around nerve fascicles has been hypothesized to result in cytotoxicity and ischemia.[18,19,20] Such effects are seen in large animal histopathologic models and may be prevented by avoiding nerve perforation.[21] Overall, peripheral nerve injuries (PNIs) are complicated and multifactorial (see Table 1).
Patient Factors | Surgical Factors | Anesthetic Factors |
|
|
|
Table 1. Patient, surgical, and anesthetic factors contributing to peripheral nerve injuries.[12,22,23]
The ASRA Practice Advisory on Neurologic Complications has made several recommendations.[12] First, based on both animal and human studies, it suggests that direct needle-to-nerve contact and intraneural injections be avoided.[24,25] While the clinical implications of needle-to-nerve contact are not generally clinically relevant, US-guided studies have not demonstrated a significant reduction in the incidence of this event.[26] Therefore, US alone cannot prevent trauma resulting from a needle entering a nerve and causing injury or inflammation that may be histologically present.[21] With the use of the nerve stimulator, one may suspect that the needle is intraneural if, for example, a 0.2 mA current threshold results in a motor response.[25] This finding, however, does not necessarily equate to the needle being located in an intrafasicular location. The ideal minimal threshold current that limits the incidence of intraneural injections yet still provokes a motor response is debatable. Overall, ENS has a low sensitivity but high specificity for direct nerve contact. Consequently, the advisory panel suggested that a current of <0.5 mA, which evokes a motor response, effectively indicates intraneural needle placement.[12] Other published literature has suggested a lower threshold to illicit concern for intraneural needle placement (closer to 0.2 mA) but suggest that higher thresholds should be considered in certain patient populations (ie, those with diabetes, multiple sclerosis and obesity).[11,25] Therefore, nerve stimulation technique may not be reliable in patients with these conditions. This finding supports the argument that more than one technique should be used to help prevent nerve injury when performing nerve block (dual-endpoint technique). Currently, no human data supports the superiority of one localization technique over another to reduce potential nerve injuries (PNIs).[12] PNIs occur with a similar frequency whether US guidance or ENS is used as the primary nerve localization tool – 2 to 4/10000 blocks.[12,23] Such outcomes may be related to variation in operator technique, poor image resolution, or attempting to inject directly adjacent to a nerve despite a lack of sonographic evidence of tissue expansion with local anesthetic injection – all potentially unsafe regional anesthesia practices.[27] Recently, we have suggested the use of a multimodal-endpoint technique when performing nerve blocks, which includes the combination of US, nerve stimulator, injection pressure monitoring, and patient feedback.17 As there is no single modality that prevents direct needle-to-nerve contact, our suggestion is in line with the practice advisory panel.[12,14]
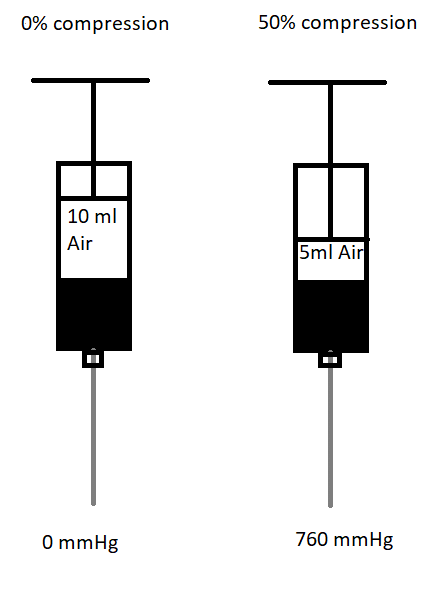
Figure 1. Figure showing the compressed air injection technique.
Ten ml of air was aspirated into the syringe and then the air column is compressed to 50% of the volume. Applying Boyle’s law (pressure x volume = constant), the pressure will double to 760 mmHg (1atm
To promote the use of a multimodal-endpoint technique, several adjustments can be made that do not add to the procedural time. As mentioned earlier, for nerve stimulation, D5W, rather than LA/NS, as the initial injectate is necessary to confirm the correct positioning of the needle. The current threshold for nerve stimulation that limits intraneural injections is significantly less than what is generally utilized for nerve localization. For nerve localization, the nerve stimulator is often set to 1 mA and, as the needle approaches the target nerve, the current is reduced until it reaches 0.5 mA or less. The elicitation of motor response at this current amplitude suggests that the needle is correctly positioned adjacent to the nerve. However, when using nerve stimulation for preventing needle-to-nerve contact, the nerve stimulator is initially set at 0.2 or 0.3 mA and the aim is not to elicit any motor response since a motor response signifies that the needle is potentially intraneural. For pressure monitoring, limiting injection pressure to <15 psi through the use of commercially available devices or compressed air injection techniques can also be used.[28,29] The compressed air injection technique (CAIT) applies the principle of Boyle’s law to allow for a simple, real-time, subjective injection pressure monitoring. When a column of air is aspirated above the column of fluid and then compressed by ≤50%, the injection pressure is ≤760 mmHg (1 atm or 14.7 psi). (Figure 1) Furthermore, we also advocate all PNBs be performed with insulated nerve block needles on awake patients for real-time feedback.
Conclusion
Despite countless studies attempting to outline differences in outcomes between US and ENS in regional anesthesia, neither technique is reliable on its own. Rather, when used together these modalities are complementary to each other and increase the likelihood that intraneural injections will be detected and prevented.[27] At the University of Alberta Hospital, we utilize a combination of US for visual guidance and low current thresholds via ENS that attempt to produce an absence of motor response, objectively monitor the injection pressure, and maintain real-time feedback from patients in an effort to increase our ability to safely perform peripheral nerve blocks.[12,30]
References
- Bresadola M. Medicine and science in the life of Luigi Galvani (1737-1798). Brain Res Bull. 1998;46(5):367-80.
- Galvani L. De viribus electricitatis in motu musculari commentarius. Bon Sci Art Inst Acad Comm. 1791;7:363-418.
- Piccolino M. Animal electricity and the birth of electrophysiology: the legacy of Luigi Galvani. Hist Neurosci. 1998;46(5):381-407.
- Klein KM, Melton SM, Grill WM, et al. Peripheral nerve stimulation in regional anesthesia. Reg Anesth Pain Med. 2012;37(4):383-92.
- Mariano ER, Marshall ZJ, Urman RD, et al. Ultrasound and its evolution in perioperative regional anesthesia and analgesia. Best Pract Res Clin Anaesthesio.l 2014;28(1):29-39.
- La Grange P, Foster PA, Pretorius LK. Application of the doppler ultrasound bloodflow detector in supraclavicular brachial plexus block. Br J Anaesth. 1978;50(9):965-7.
- Albrecht E, Chin KJ. Advances in regional anesthesia and acute pain management: a narrative review. Anaesthesia. 2020;75(Suppl 1):e101-10.
- Winnie AP, Collins VJ. The subclavian perivascular technique of brachial plexus anesthesia. Anesthesiology. 1964 May-Jun;25:353-63.
- Ligouri GA, Zayas VM, YaDeau JT, et al. Nerve localization techniques for interscalene brachial plexus blockade: a prospective, randomized comparison of mechanical paresthesia versus electrical stimulation. Anesth Analg. 2006;103:761-67.
- Kiefer RT. Eliciting paresthesias for peripheral nerve block: a harmful clinical standard? Anesth Analg. 2001;92(3):795.
- Stan TC, Krantz MA, Solomon DL, et al. The incidence of neurovascular complications following axillary brachial plexus block using a transarterial approach: a prospective study of 1000 consecutive patients. Reg Anesth. 1995;20:486-92.
- Neal JM, Barrington MJ, Brull R, et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine. Reg Anesth Pain Med. 2015;40(5):401-30.
- Andres JD, Alonso-Inigo JM, Sala-Blanch X, et al. Nerve stimulation in regional anesthesia: theory and practice. Best Pract Res Clin Anaesthesio.l 2005;19(2):153-74.
- Tsui BCH, Chan V, Finucane BT et al. Electrical nerve stimulation. In: Tsui BCH, Chan V, Finucane BT et al. Atlas of ultrasound and nerve stimulation-guided regional anesthesia. New York, NY: Springer Science+Business Media 2007:9-20.
- Tsui BCH, Kropelin B. The electrophysiological effect of dextrose 5% in water on single-shot peripheral nerve stimulation. Anesth Analg. 2005;100(6):1837-9.
- Tsui BCH, Wagner A, Finucane A. Electrophysiologic effect of injectates on peripheral nerve stimulation. Reg Anesth Pain Med. 2004;29:189-93.
- Retter S, Szerb J, Kwofie K et al. Incidence of sub-perineural injection using a targeted intracluster supraclavicular ultrasound-guided approach in cadavers. Br J Anaesth. 2019;122(6):776-81.
- Hara K, Sakura S, Yokokawa N, et al. Incidence and effects of unintentional intraneural injection during ultrasound-guided subgluteal sciatic nerve block. Reg Anesth Pain Med. 2012;37(3):289-93.
- Choquet O, Noble GB, Abbal B, et al. Subparaneural Versus Circumferential Extraneural Injection at the Bifurcation Level in Ultrasound-Guided Popliteal Sciatic Nerve Blocks: A Prospective, Randomized, Double-Blind Study. Reg Anesth Pain Med. 2014;39(4):306-11.
- Cappelleri G, Ambrosoli AL, Gemma M, et al. Intraneural ultrasound-guided sciatic nerve block: minimum effective volume and electrophysiologic effects. Anesthesiology. 2018;129(2):241-48.
- Wiesmann T, Steinfeldt T, Exner M, et al. Intraneural injection of a test dose of local anesthetic in peripheral nerves. Acta Anaesthesiol Scand. 2017;61:91-8.
- Welch MB, Brummett CM, Welch TD, et al. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111:490-97.
- Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS regional anesthesia hotline service. Anesthesiology. 2002;97:1274-80.
- Steinfeldt T, Poeschle S, Nimphius W, et al. Forced needle advancement during needle-nerve contact in a porcine model: histologic outcome. Anesth Analg. 2011;113:417-20.
- Bigeleisen PE, Groen G, Moayeri Nizar. Ultrasound-guided supraclavicular block: what is intraneural? Anesthesiology. 2010;112:251-52.
- Neal JM. Ultrasound-guided regional anesthesia and patient safety: an evidence-based analysis. Reg Anesth Pain Med. 2010;35:S59–S67.
- Ip VHY, Ozelsel TJP, Sondekoppam RV et al. Multimodal monitoring approach: the key to safe performance of peripheral nerve blocks. Br J Anaesth. 2019;123(3):e469-70.
- Tsui BCH, Knezevich MP, Pillay JJ. Reduced injection pressure using a compressed air injection technique (CAIT): an in vitro study. Reg Anesth Pain Med. 2008;33:168-73.
- Hadzic A, Dilberovic F, Shah S et al. Combination of intraneural injection and high injection pressure leads to fascicular injury and neurologic deficits in dogs. Reg Anesth Pain Med. 2004;29(5):417-23.
- Ip VH, Tsui BC. Practical concepts in the monitoring of injection pressures during peripheral nerve blocks. Int Anesthesiol Clin. 2011;49(4):67-80.
Leave a commentOrder by
Newest on top Oldest on top