How I Do It: Ultrasound-Guided Temporomandibular Dysfunction Prolotherapy
Cite as: Louw WF. How I do it: ultrasound-guided temporomandibular dysfunction prolotherapy. ASRA News. 2021;46. https://doi.org/10.52211/asra050121.026.
Introduction
Temporomandibular dysfunction (TMD) is a chronic disease resulting in considerable joint pain, dysfunction, and interference with activities of daily living. Temporomandibular dysfunction affects up to 25% of adults in some studies.1
TMD remains a recurrent or persistent condition in more than 50% of diagnosed cases at 5-year follow-up2,3,4 and consists of a combination of myofascial, degenerative joint pathology as well as disc dysfunction etiologies.
In my chronic pain practice, I see many patients every week who have had disappointing results with pharmacotherapy or physiotherapy, have seen multiple specialists without much improvement, including occlusal splints and multiple corticosteroid injections, and who have had needless surgical procedures done. A case that springs to mind is a 20-year-old woman who has had bilateral temporomandibular joint (TMJ) replacements done without much benefit in pain. Considering that surgical management should be used as a last resort, a conservative treatment approach that is reliable and cost-effective is needed.5
Over the last 8 years, I have been using dextrose prolotherapy in my practice as a treatment for this condition, with more than 80% of patients reporting more than 50% improvement in pain and dysfunction long term. A double-blind, randomized controlled trial (RCT) was also performed by our group and published, with a favorable review by NEJM Journal Watch. 6,7
Patients are routinely given isometric TMJ exercises pre- and post-treatment.
Anatomy
The TMJ is a bi-arthroidal hinge joint and is the articulation between the glenoid/mandibular fossa of the temporal bone and the mandibular condyle. (Figure 1).8
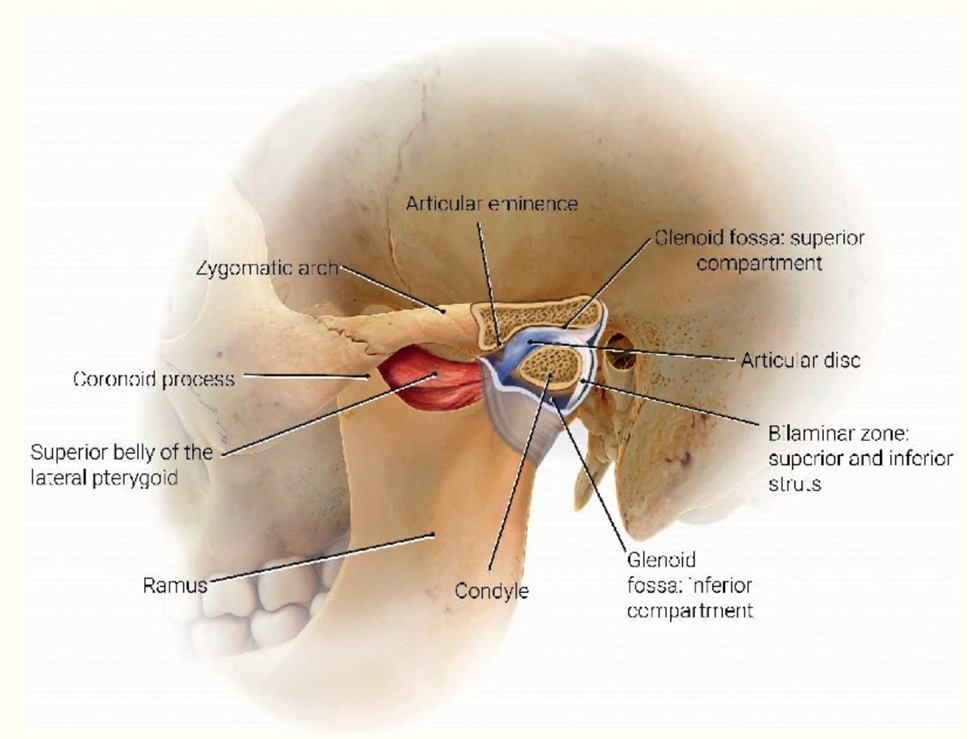
Figure 1. Anatomy (Artist: Judy Rubin)
From: Friedman SN, Grushka M, Beituni HK, Rehman M, Bressler HB, Friedman L. Advanced Ultrasound Screening for Temporomandibular Joint (TMJ) Internal Derangement. Radiol Res Pract. 2020:1809690. Published 2020 May 4. doi:10.1155/2020/1809690 (Used with permission)
The joint allows multiple complex jaw movements such as chewing, talking, and yawning.
The TMJ contains fibrocartilaginous surfaces and an articular disc, which divides the joint into superior and inferior articular cavities, lined by separate superior and inferior synovial membranes.
The joint is surrounded by a capsule, a fibrous membrane that surrounds the joint and attaches to the articular eminence, the articular disc, and the neck of the mandibular condyle.
The articular disc is attached to the condyle and to the collateral ligaments. The anterior disc attaches to the joint capsule and the superior head of the lateral pterygoid muscle. The posterior portion of the disc attaches to the mandibular fossa and is referred to as the retrodiscal tissue.
The retrodiscal tissue is highly innervated and is often a major contributor to pain generation in TMD.
Technique
The two highest yield injection sites in my experience are illustrated in Figure 2: The intra-articular compartment and the coronoid process attachment of the temporalis tendon (enthesis).9
Scanning Technique. For the intra-articular injection, the patient is placed in a side-lying position with the head turned to the opposite side. The high-frequency linear transducer is placed in a coronal orientation. (Figure 3).
Ultrasound anatomy reveals the mandibular condyle and the articular eminence of the temporal bone. (Figure 4).
An in-plane technique is used to inject 0.5 to 0,7 ml intra-articular into the synovial pouch of the inferior joint space, with injectate filling this space, exerting effects on the synovial tissues, mandibular cartilage, and retrodiscal tissue. (Figure 5).
Following this, the probe is rotated 90 degrees to a transverse orientation. The mandibular coronoid process enthesis is then injected out of plane with 0.5 ml injectant. (Figures 6 and 7).
Injectant Preparation
- 1 ml saline
- 1 ml 1 % preservative-free lidocaine
- 1 ml 50 % dextrose
This amounts to +/- 17% dextrose in 0.3 % lidocaine. An intra-articular injection with 0.5-0.7 ml injectant is administered over the coronoid process and/or masseter attachments with approximately 0.5 ml per site.
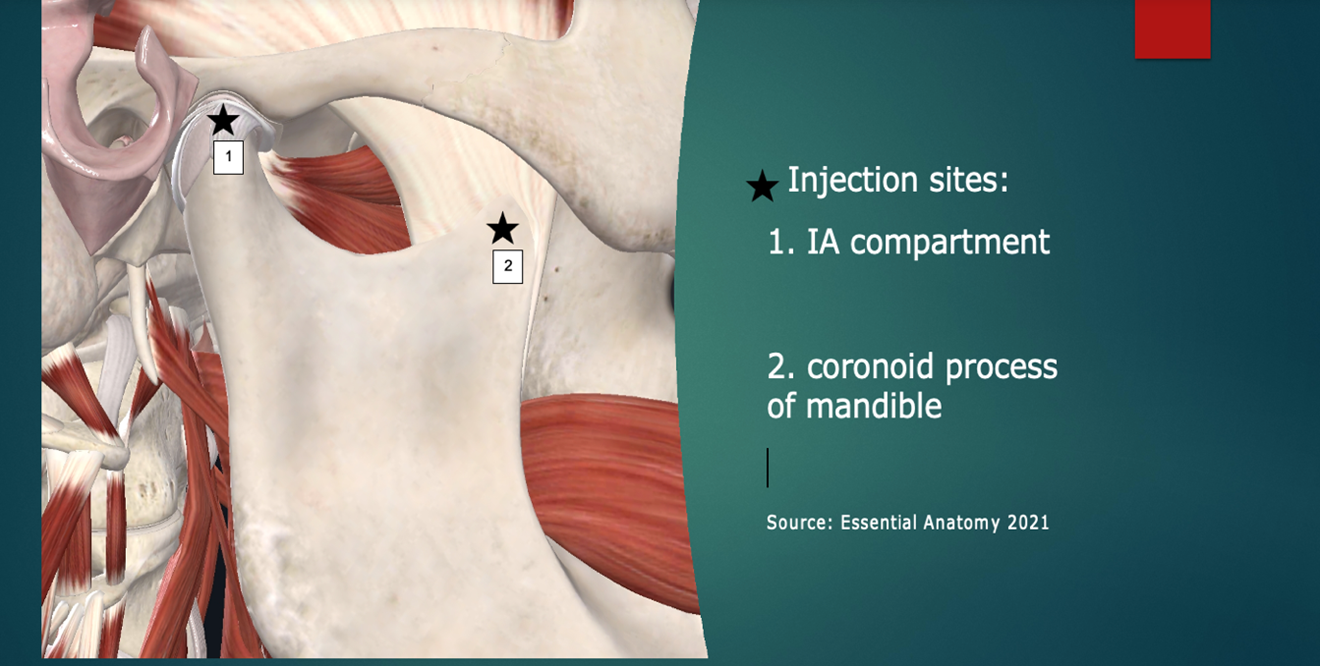
Figure 2: Injection sites (simplified “Louw Method”). Image courtesy of Complete Anatomy, https://3d4medical.com/
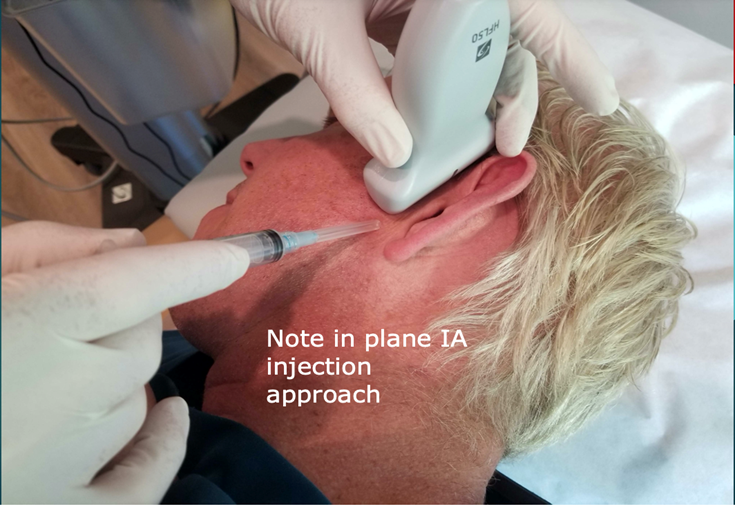
Figure 3: Scanning technique. The patient is placed in a side-lying position with the head turned to the opposite side. The high-frequency 15-6 MHz linear transducer is placed in a coronal orientation.
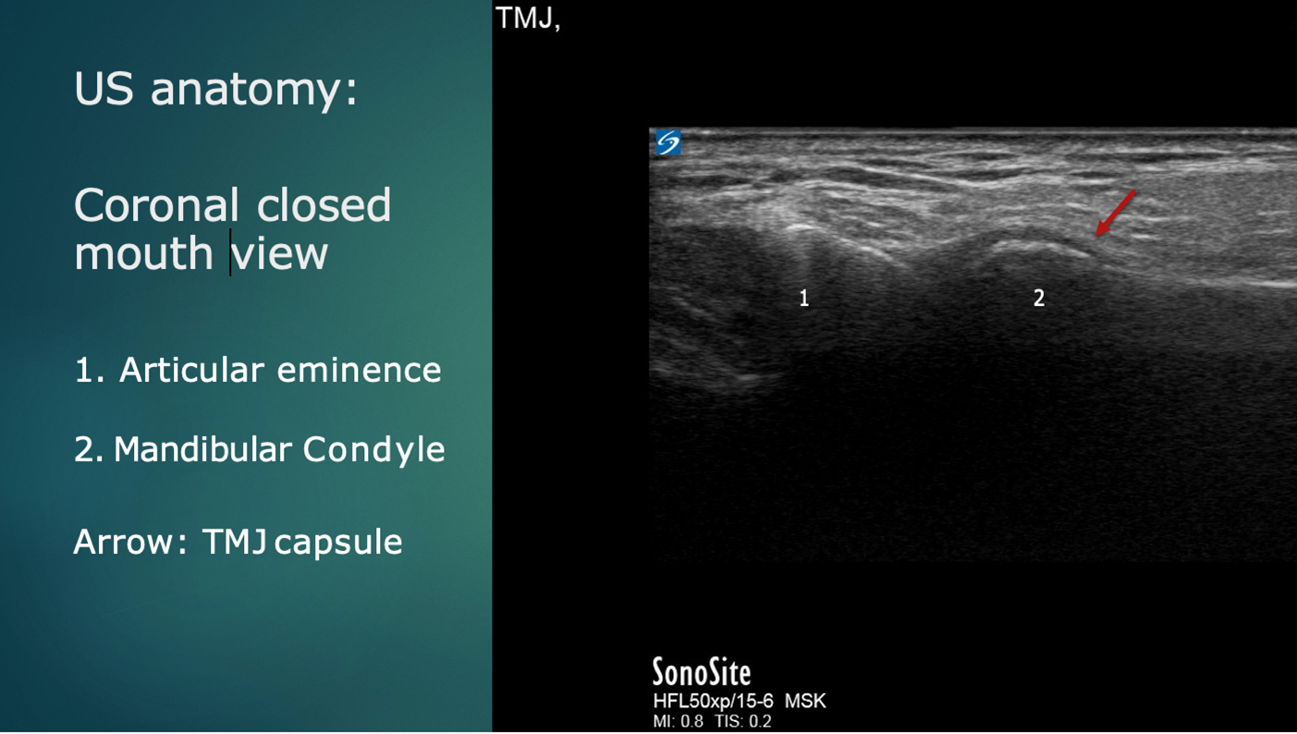
Figure 4: Ultrasound anatomy: Coronal plane
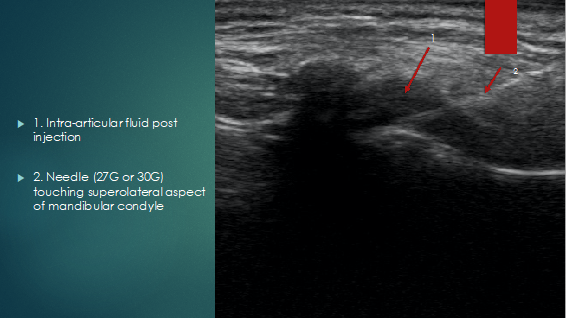
Figure 5: Coronal view post injection
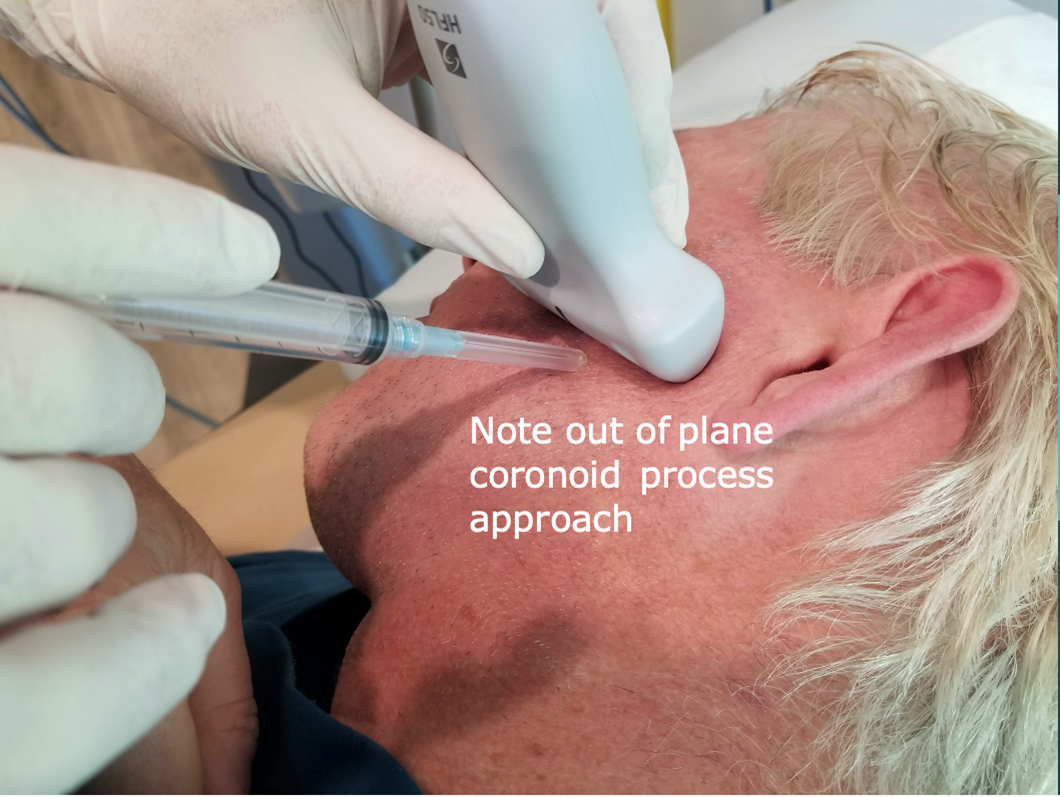
Figure 6: Injection approach: Coronoid process
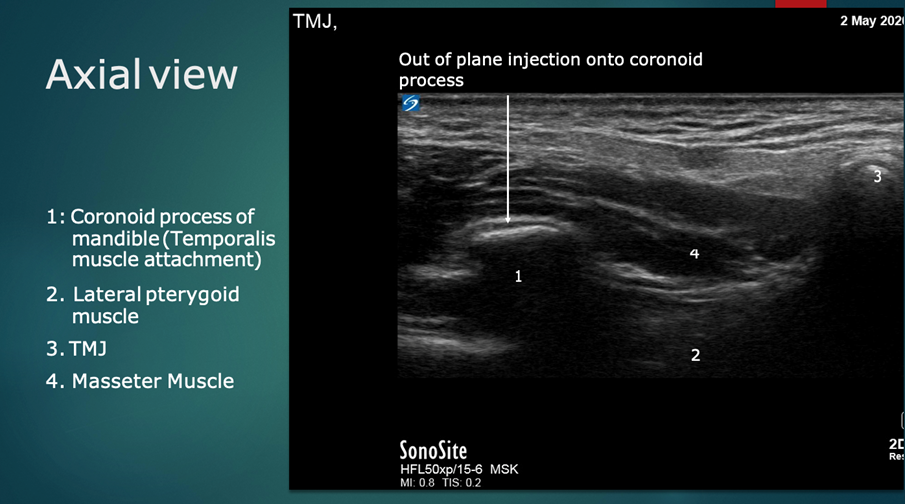
Figure 7: Ultrasound anatomy: Transverse/axial plane
Why Dextrose Prolotherapy?
Significant adverse joint events following corticosteroid injections have been reported and include accelerated osteoarthritis progression, subchondral insufficiency fracture, complications of osteonecrosis, soft tissue atrophy, increased risk of infection, and hypothalamic-pituitary-adrenal axis (HPA) suppression. These events are becoming more recognized by physicians.10
Compared to the complexity and cost associated with both botulinum toxin and platelet rich plasma injection, this injection technique stands out as being safe, elegant, well-tolerated, and reproducible. In studies that include intra-articular dextrose injections in participants with knee osteoarthritis, dextrose is implicated as an independent agent in pain reduction. Post-procedure arthroscopic evaluation further suggests a chondrogenic effect of dextrose.11 This modality can be repeated monthly, as it does not lead to degradation of cartilage or atrophy of connective tissues, as seen with corticosteroid injections.
A therapeutic benefit of dextrose based on a neurogenic mechanism was proposed in 2008 12 and is supported by recent RCTs and open-label studies using dextrose hydrodissection for carpal tunnel syndrome, peritendinous dextrose injection for Achilles tendinopathy, and epidural dextrose for chronic low back pain.13
Similar effects have been reported in a retrospective analysis of the results from regional hydrodissection using dextrose in patients with various neurogenic pain conditions of the upper body.14
Neural Mechanism
Several neural mechanisms have been hypothesized. First, downregulation of the transient receptor potential vanilloid (TRPV) ion channel is a primary therapeutic target in chronic pain management.15 A class effect of sugars resulting in indirect downregulation of the effects of TRPV receptor 1 ion channel activation has been proposed based on an RCT using a polyol (mannitol) with structural similarity to dextrose.16
Second, in vitro nociceptive C fibers in corneal explants fire faster in the presence of hypoglycemia, followed by a prompt reduction in firing rate with correction of the hypoglycemic state.17 This suggests that a relative hypoglycemia of high-energy cells (nerves) may hypopolarize pain-producing C fibers, lowering the threshold of stimuli required for depolarization and resultant pain perception.
Third, coadministration of 5% dextrose to reduce pain upon infusion of pain-inducing chemotherapeutic agents or microspheres18-20 may point to a hyperpolarization effect of dextrose on the cell membrane of pain-producing C fibers, increasing the threshold required for depolarization.
Summary
Ultrasound-guided dextrose prolotherapy is a conservative treatment approach which is reliable, cost-effective, and reproducible for TMD in particular, and should be used more widely in interventional pain practices.
See these suggested home exercises for patients.

W. Francois Louw, CCFP(EM), FCFP, MBChB (Pret), DA(SA), ECFMG, Adv. Dipl Pain Mgt CAPM (Interventional Pain Management), is a physician at Bill Nelems Pain and Research Centre in Kelowna, British Columbia, Canada. Dr. Louw is a clinical associate professor at the University of British Columbia department of Family Practice.
References
- Liu F, Steinkeler A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent Clin North Am. 2013;57(3):465-479. https://doi.org/10.1016/j.cden.2013.04.006.
- Maixner W, Diatchenko L, Dubner R, et al. Orofacial pain prospective evaluation and risk assessment study- the OPPERA study. J Pain. 2011;12(11, suppl):T4-T11. e11-e12. https://doi.org/10.1016/j.jpain.2011.08.002.
- Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74(2-3):315-326. https://doi.org/10.1016/s0304-3959(97)00194-2.
- Rammelsberg P, LeResche L, Dworkin S, Manci L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17(1):9-20
- Rajapakse S, Ahmed N, Sidebottom AJ. Current thinking about the management of dysfunction of the temporomandibular joint: a review. Br J Oral Maxillofac Surg. 2017;55(4):351-356. https://doi.org/10.1016/j.bjoms.2016.06.027.
- Louw WF et al. Treatment of temporomandibular dysfunction with hypertonic dextrose injection (prolotherapy): A randomized controlled trial with long-term partial crossover. Mayo Clin Proc 2019 May; 94:820. https://doi.org/10.1016/j.mayocp.2018.07.023.
- Brett AS. “Prolotherapy” for temporomandibular joint dysfunction. NEJM Journal Watch. 2019, May 16. Available at: https://www.jwatch.org/na49146/2019/05/16/prolotherapy-temporomandibular-joint-dysfunction
- Friedman SN, Grushka M, Beituni HK, Rehman M, Bressler HB, Friedman L. Advanced ultrasound screening for temporomandibular joint (TMJ) internal derangement. Radiol Res Pract. 2020:1809690. https://doi.org/10.1155/2020/1809690.
- 3D4Medical. Complete anatomy 2001. Available at: https://3d4medical.com/
- Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293(3):656-663. https://doi.org/10.1148/radiol.2019190341.
- Topol GA, Podesta LA, Reeves KD, et al. The chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. PM R. 2016;8(11):1072-1082. https://doi.org/10.1016/j.pmrj.2016.03.008.
- Lyftogt J. Pain conundrums: which hypothesis? Central nervous system sensitization versus peripheral nervous system autonomy. Australas Musculoskel Med. 2008;13(11):72-74.
- Maniquis-Smigel L, Reeves KD, Rosen JH, et al. Analgesic effect and potential cumulative benefit from caudal epidural D5W in consecutive participants with chronic low back and buttock/leg pain [published online ahead of print June 8, 2018]. J Altern Complement Med. 24(12):1189-1196. https://doi.org/10.1089/acm.2018.0085.
- Lam SKH, Reeves KD, Cheng A-L. Transition from deep regional blocks toward deep nerve hydrodissection in the upper body and torso: method description and results from a retrospective chart review of the analgesic effect of 5% dextrose water as the primary hydrodissection injectate. Biomed Res Int. 2017; 1-17. https://doi.org/10.1155/2017/7920438.
- Malek N, Pajak A, Kolosowska N, Kucharczyk M, Starowicz K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci. 2015;65:1-10. https://doi.org/10.1016/j.mcn.2015.02.001.
- Bertrand H, Kyriazis M, Reeves KD, Lyftogt J, Rabago D. Topical mannitol reduces capsaicin-induced pain: results of a pilot level, double-blind randomized controlled trial. PM R. 2015;7(11): 1111-1117. https://doi.org/10.1016/j.pmrj.2015.05.002.
- MacIver MB, Tanelian DL. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology. 1992;76(4):617-623.
- Hosokawa A, Nakashima T, Ogawa Y, Kozawa K, Kiba T. Coadministration of 5% glucose solution relieves vascular pain in the patients administered gemcitabine immediately. J Oncol Pharm Pract. 2013;19(2):190-192. https://doi.org/10.1177/1078155212449679.
- Nakashima T, Ogawa Y, Kimura A, et al. Coadministration of 5% glucose solution has a decrease in bendamustine related vascular pain grade. J Oncol Pharm Pract. 2012; 18(4):445-447. https://doi.org/10.1177/1078155212442560.
- Paprottka KJ, Lehner A, Fendler WP, et al. Reduced periprocedural analgesia after replacement of water for injection with glucose 5% solution as the infusion medium for 90Y-Resin microspheres. J Nucl Med. 2016;57(11):1679-1684. https://doi.org/10.2967/jnumed.115.170779.
Leave a commentOrder by
Newest on top Oldest on top