POCUS Spotlight: Airway Ultrasound
Cite as: Kolli S, Singh M. POCUS spotlight: airway. ASRA News. 2021;46. https://doi.org/10.52211/asra080121.046.
Introduction
In the last two decades, clinical application of ultrasound technology has expanded, not only for visualization of anatomical structures and identification of pathology, but also to facilitate medical procedures. Recently, the use of point-of-care-ultrasound (POCUS) has gained popularity for a variety of perioperative procedures and guidance in high-acuity settings. Another area of interest is the use of POCUS for evaluation of upper airway structures. This has proved to be a valuable and noninvasive tool for anesthesiologists, critical care and emergency physicians. Training and understanding of applied airway sonoanatomy will enable physicians to identify abnormal or difficult airway and perform procedures such as percutaneous cricothyroidotomy or tracheostomy, confirmation of endotracheal tube (ETT) placement, and performance of regional blocks for airway management.
Training and understanding of applied airway sonoanatomy will enable physicians to identify abnormal or difficult airway and perform procedures such as percutaneous cricothyroidotomy or tracheostomy, confirmation of endotracheal tube placement, and
performance of regional blocks for airway management.
Sonoanatomy of the Upper Airway
The important airway structures that can be visualized using ultrasound are tongue, hyoid bone, epiglottis, thyrohyoid membrane, thyroid cartilage, cricothyroid membrane, cricoid cartilage, trachea, and esophagus.1 (Table 1, Figures 1-9) The POCUS airway scanning technique pertains to dividing the upper airway structures into suprahyoid and infrahyoid areas. A standard linear high frequency transducer (13-6 MHz) is sufficient to scan infrahyoid airway structures while the low frequency curved (3-8 MHz) is more suitable for tongue and deeper suprahyoid structures. Patient should be lying supine with the head extended and neck flexed in the sniffing position for scanning suprahyoid structures, and neck extended for infrahyoid structures. In this article, we will discuss the indications of POCUS airway technique with a specific focus on identification of the cricothyroid membrane and performance of airway blocks for facilitation of airway management in an awake patient.
Table 1: Sonographic Appearance of Airway Structures (Video 1)
| Anatomical Structure | Probe Position and Image Acquisition | Sonographic Appearance |
| Tongue | Midsagittal, below the mentum | Fan shaped with intrinsic muscles giving striated appearance; hyperechoic line of dorsal surface from A-M interface (Figure 2) |
| Hyoid bone | Transverse over the neck; slide caudad from the mentum | Superficial hyperechoic inverted U-shaped linear structure (Figure 3) |
| Sagittal or parasagittal | Narrow curved structure, casting acoustic shadow (Figure 2) | |
| Epiglottis | Transverse above the thyroid cartilage through the thyrohyoid membrane window | Hypoechoic transverse curvilinear structure, with hyperechoic pre-epiglottic space and bright A-M interface delineating the posterior border (Figure 4) |
| Sagittal in most subjects | Hypoechoic longitudinal curvilinear structure | |
| Thyrohyoid membrane | Transverse between the hyoid bone and the thyroid cartilage | Hyperechoic line between the hyoid bone and thyroid cartilage |
| Parasagittal | Hyperechoic line between the hyoid bone and thyroid cartilage (Figure 5) | |
| Thyroid cartilage | Transverse on thyroid prominence | Inverted V-shaped hypoechoic, with varying age-related hyperechogenicity and intraglottic structures (Figure 6) |
| Parasagittal over the thyroid cartilage | Linear hypoechoic structure with varying calcification (Figure 5) | |
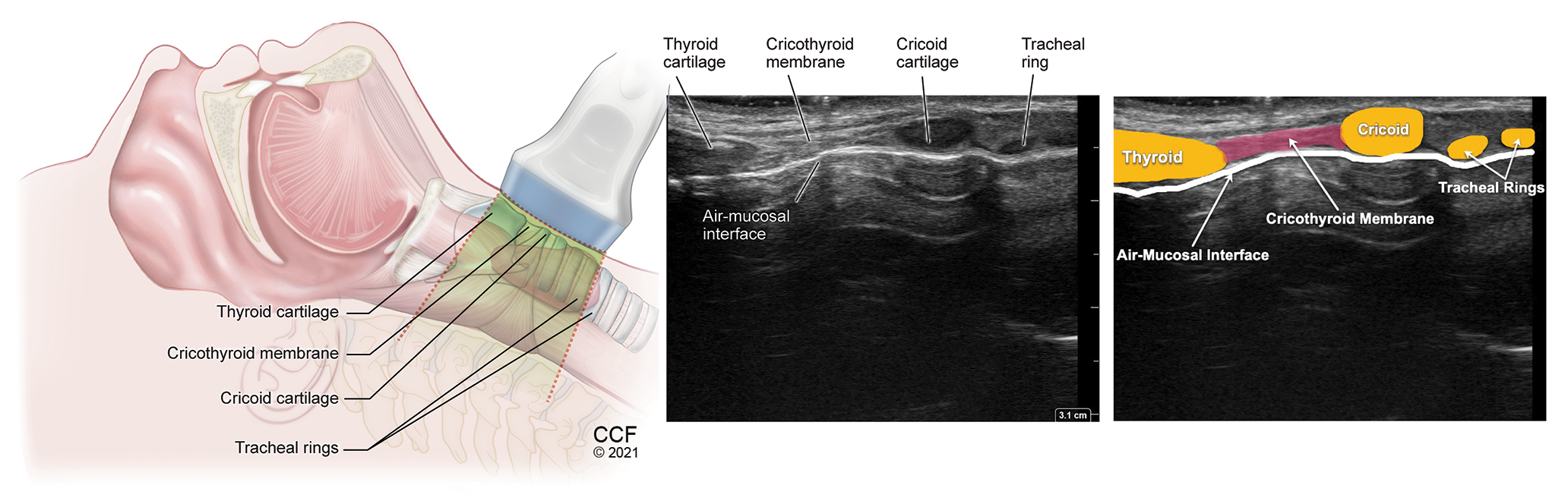
| Cricothyroid membrane | Transverse using TACA technique (Video 2) | Hyperechoic transverse line between the thyroid and cricoid cartilages (Figure 7) |
| Midsagittal probe above the suprasternal notch and slide cephalad (Video 3) | Longitudinal hyperechoic line between thyroid and cricoid cartilages. (Figure 10) | |
| Cricoid cartilage | Transverse over the cricoid cartilage | Superficial, thick hypoechoic arch-shaped structure (Figure 8) |
| Sagittal, probe above the suprasternal notch | Hypoechoic oval structure larger than the tracheal rings (Figure 10) | |
| Trachea | Transverse over the suprasternal notch | Inverted U-shaped hypoechoic rings on transverse view (Figure 9) |
| Midsagittal over the suprasternal notch | Hypoechoic oval structures (string of pearls/beads appearance) (Figure 10) | |
| Esophagus | Transverse over the suprasternal notch sliding laterally (usually to left) | Collapsed muscular structure lateral to trachea; swallowing facilitates its identification (Video 4) |
Patient positioned supine with head and neck extended, use low frequency, curved transducer for suprahyoid structures and high frequency, linear transducer for hyoid and infrahyoid structures.
A-M – air-mucosal; TACA – (Thyroid Cartilage - Air line - Cricoid Cartilage - Airline)
Video 1. Transverse Scan of the Upper Airway
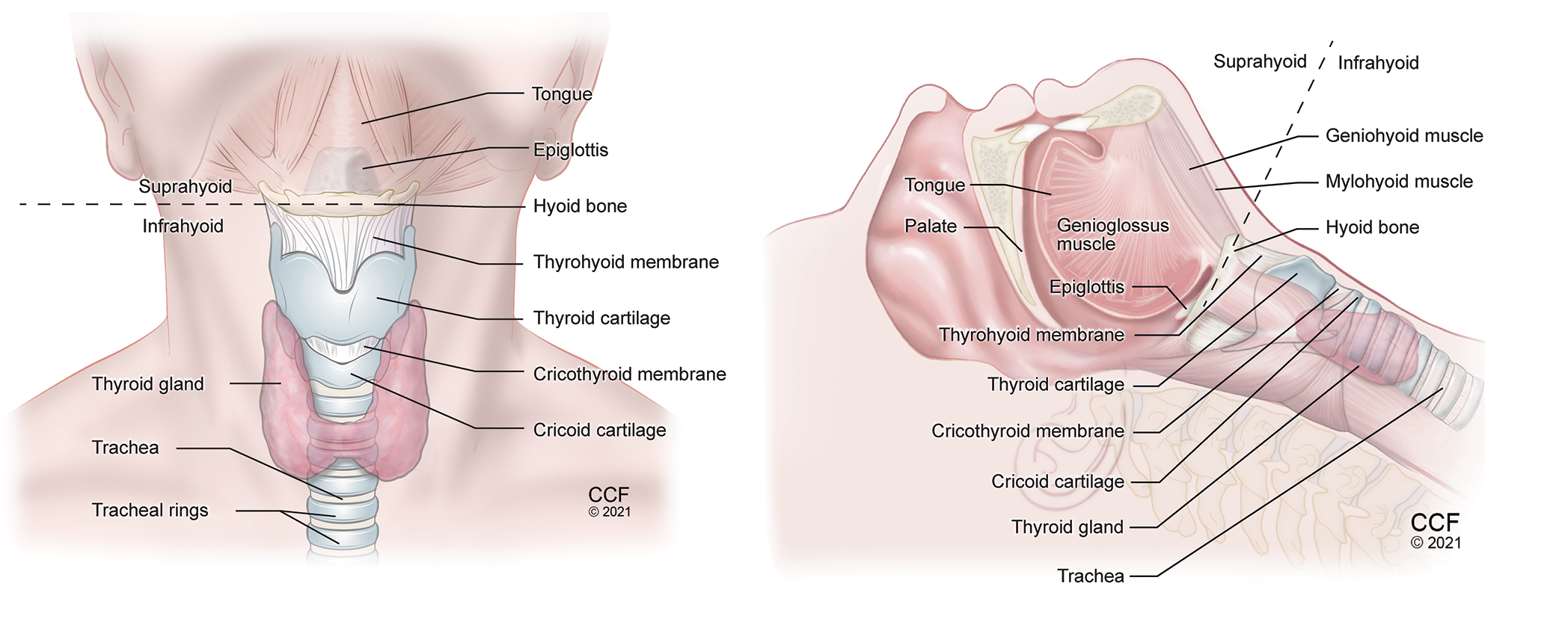 Figure 1. Anatomy of the neck anterior view (A) and lateral view (B) showing important airway structures.
Figure 1. Anatomy of the neck anterior view (A) and lateral view (B) showing important airway structures.
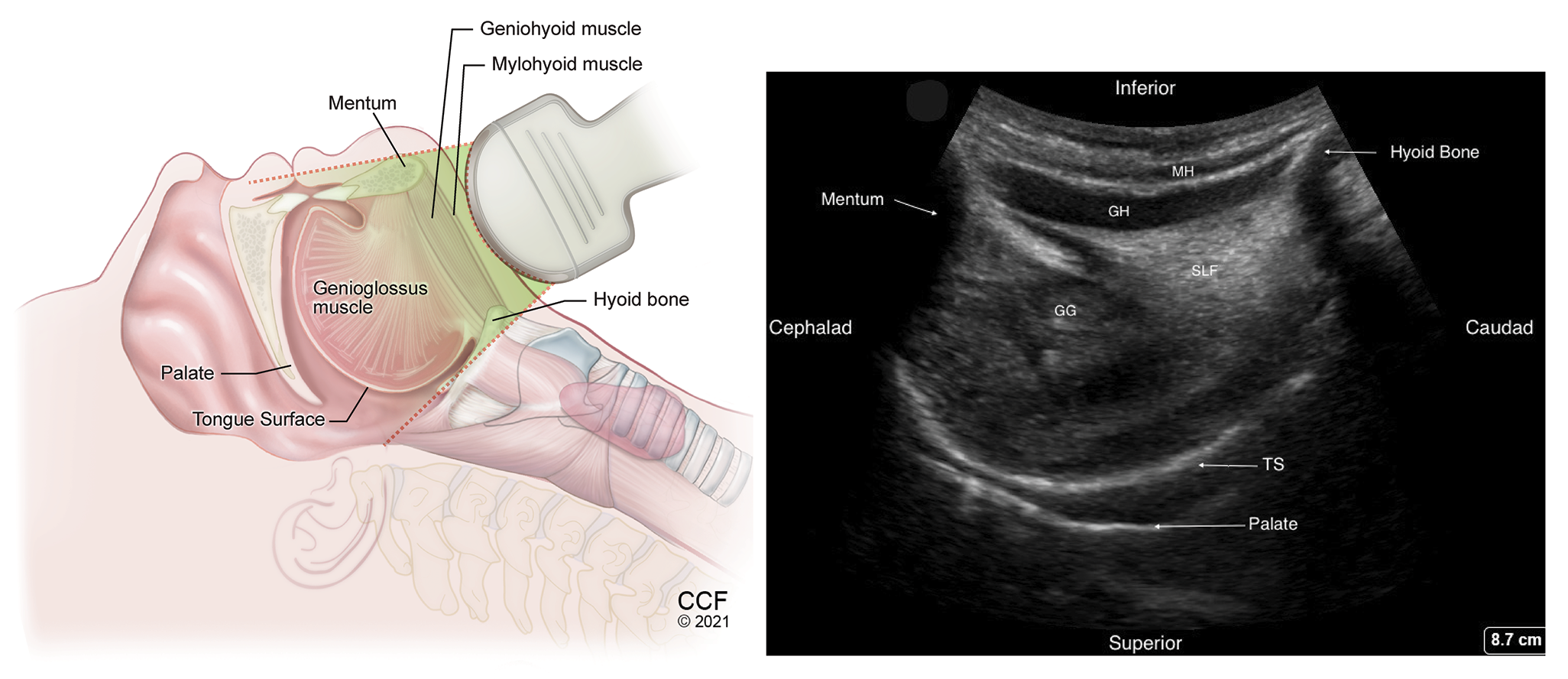 Figure 2. Sagittal view of the suprahyoid structures using low frequency transducer, placed in the submandibular area behind the mentum.
Figure 2. Sagittal view of the suprahyoid structures using low frequency transducer, placed in the submandibular area behind the mentum.
MH -Mylohyoid muscle, GH – Geniohyoid muscle, GG – Genioglossus muscle, TS – Dorsal tongue surface, SLF – Sublingual fat.
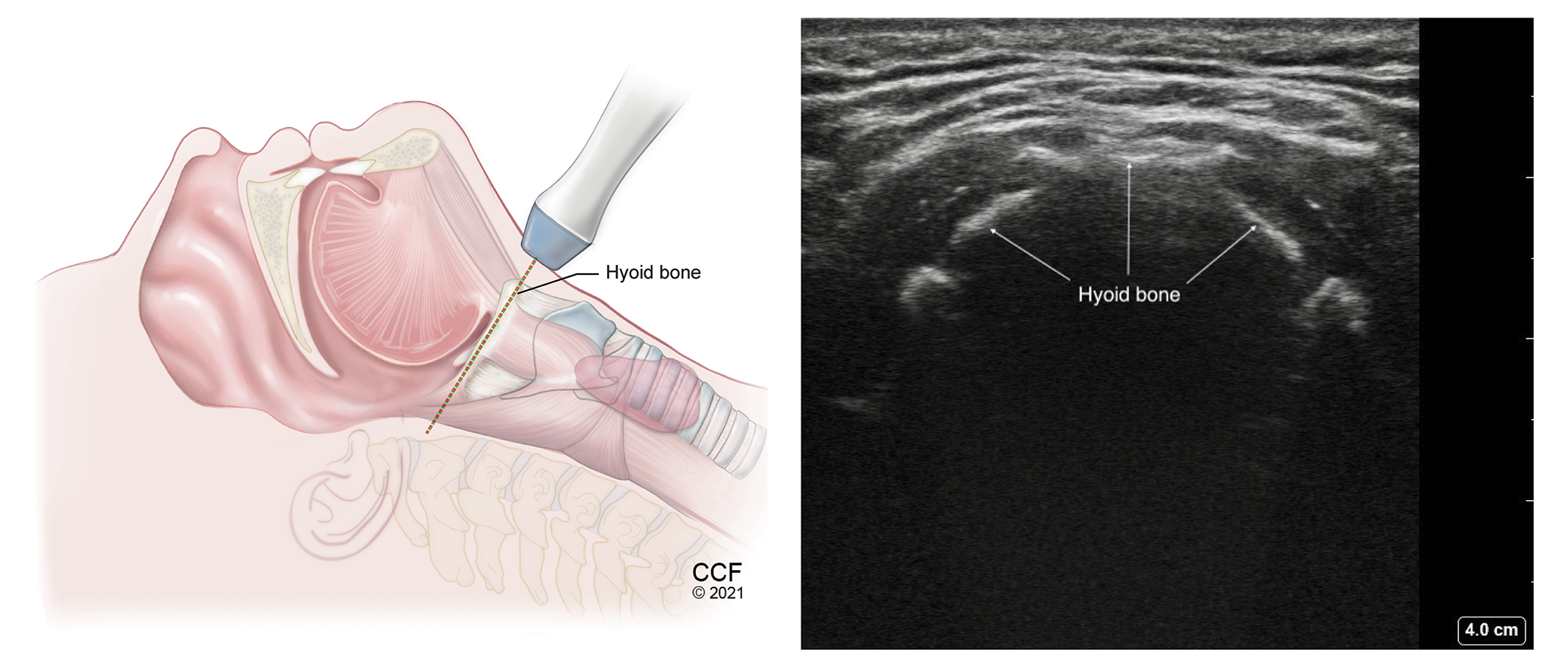 Figure 3.
Transverse view of the hyoid bone with high frequency linear transducer placed over the hyoid bone.
Figure 3.
Transverse view of the hyoid bone with high frequency linear transducer placed over the hyoid bone.
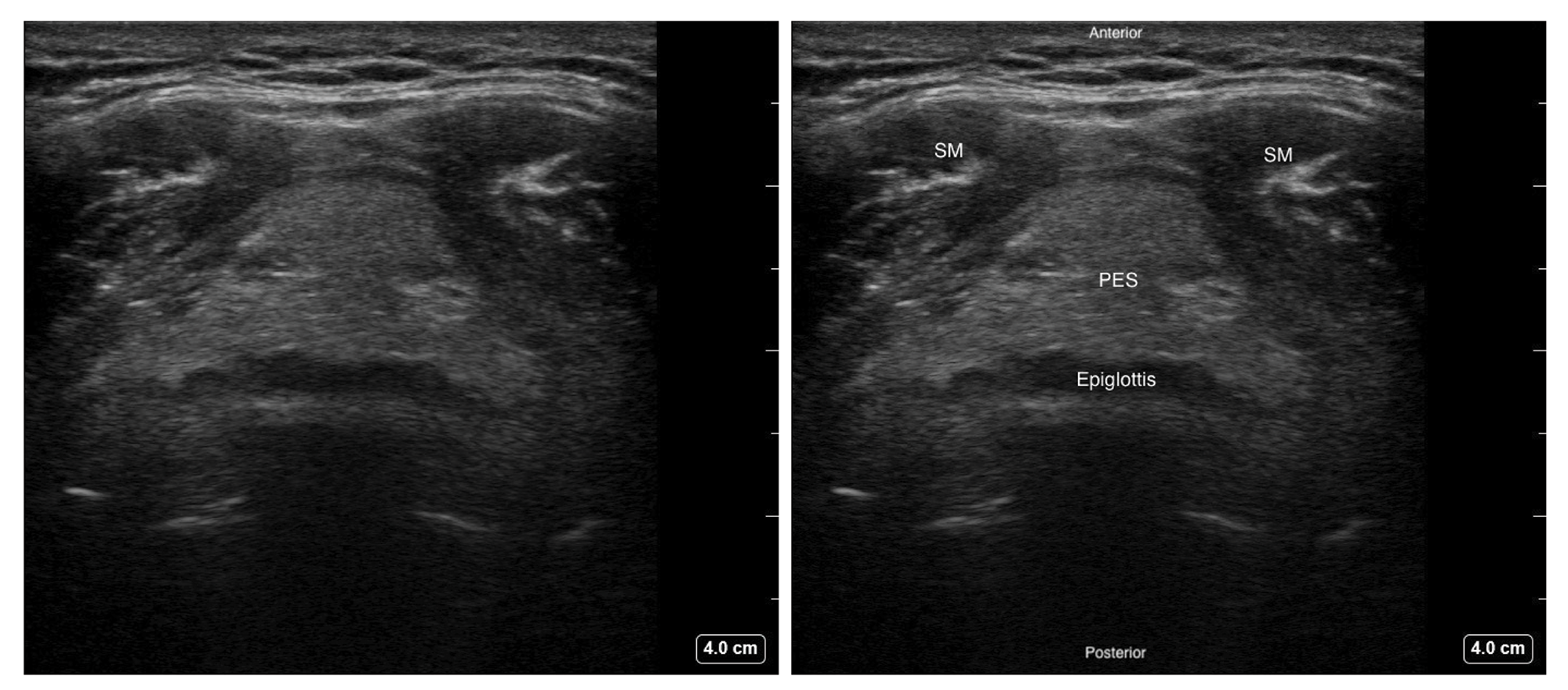 Figure 4. Transverse view of the epiglottis with high frequency linear transducer over the thyrohyoid area.
Figure 4. Transverse view of the epiglottis with high frequency linear transducer over the thyrohyoid area.
SM - Strap muscle, PES - Pre-epiglottic space
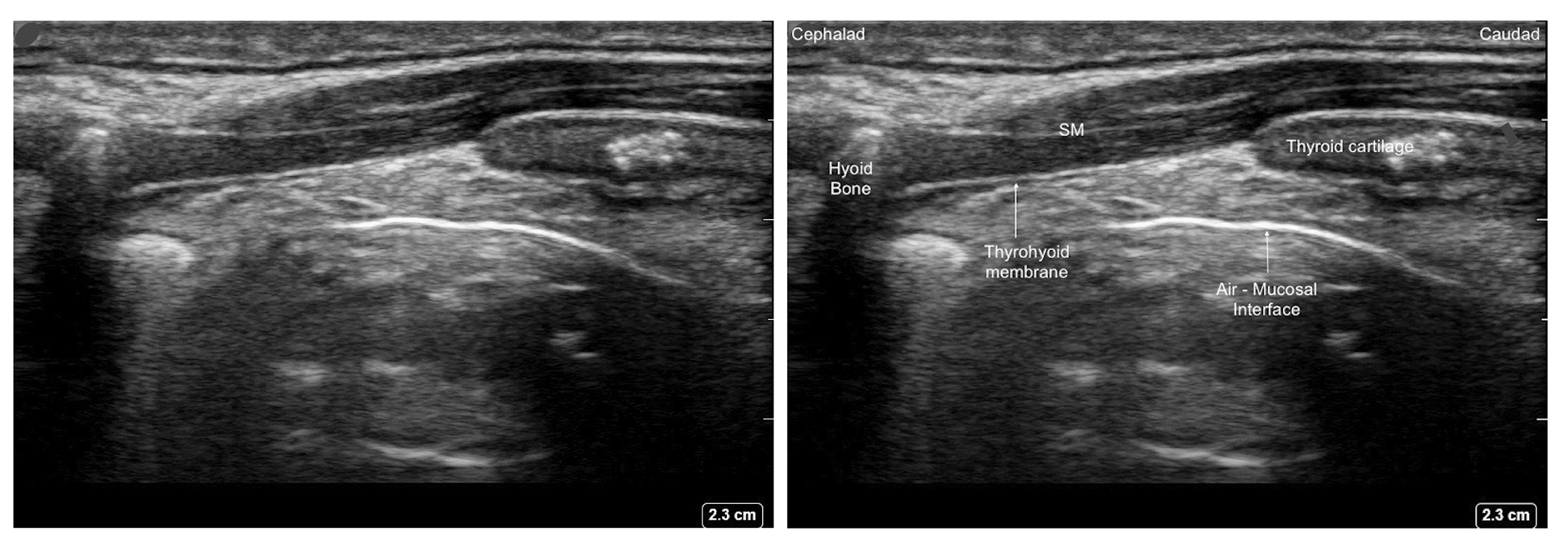 Figure 5. Parasagittal view of the thyrohyoid membrane with high frequency linear transducer.
Figure 5. Parasagittal view of the thyrohyoid membrane with high frequency linear transducer.
SM - Strap muscle
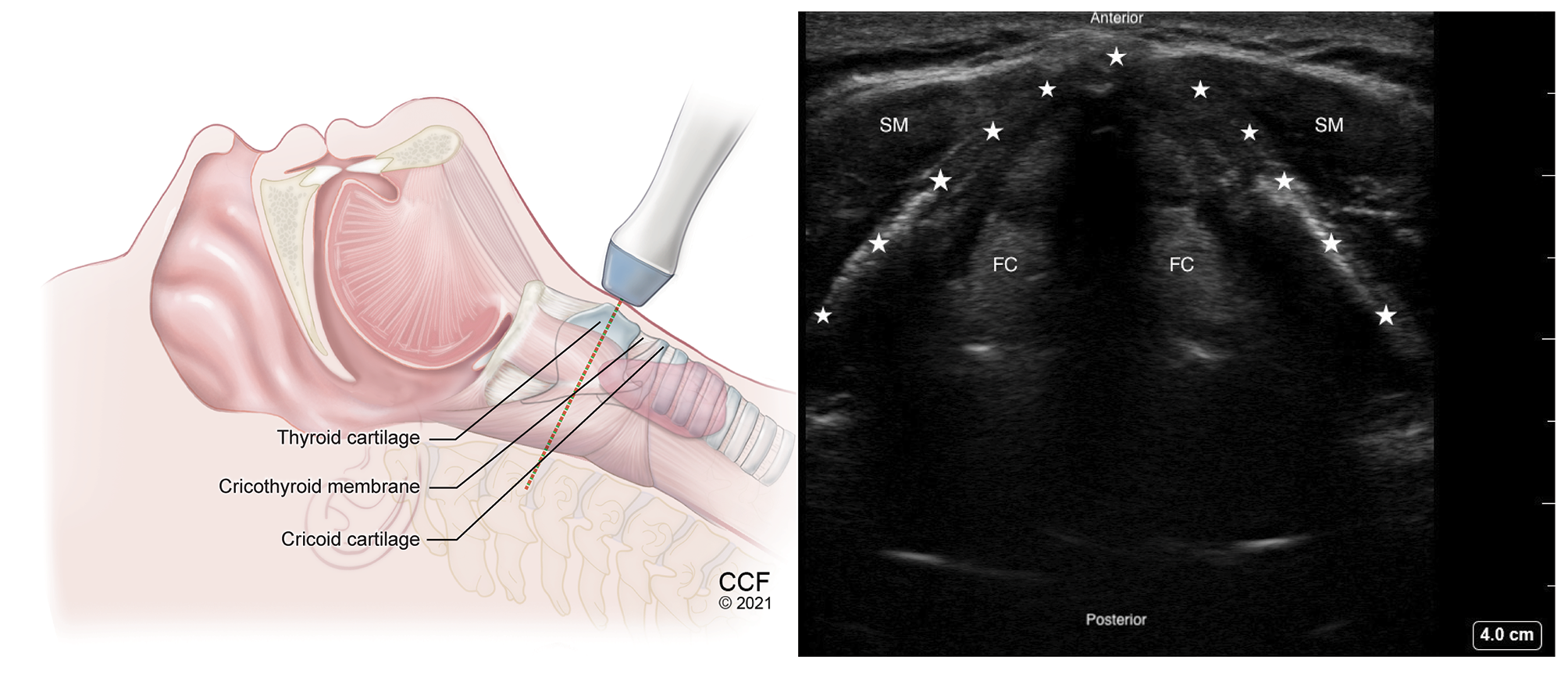 Figure 6. Transverse view of thyroid cartilage with high frequency linear transducer over the thyroid prominence.
Figure 6. Transverse view of thyroid cartilage with high frequency linear transducer over the thyroid prominence.
SM - Strap muscle, FC - False Cords. Thyroid cartilage inverted V-shaped, marked by stars.
Video 2. TACA Technique
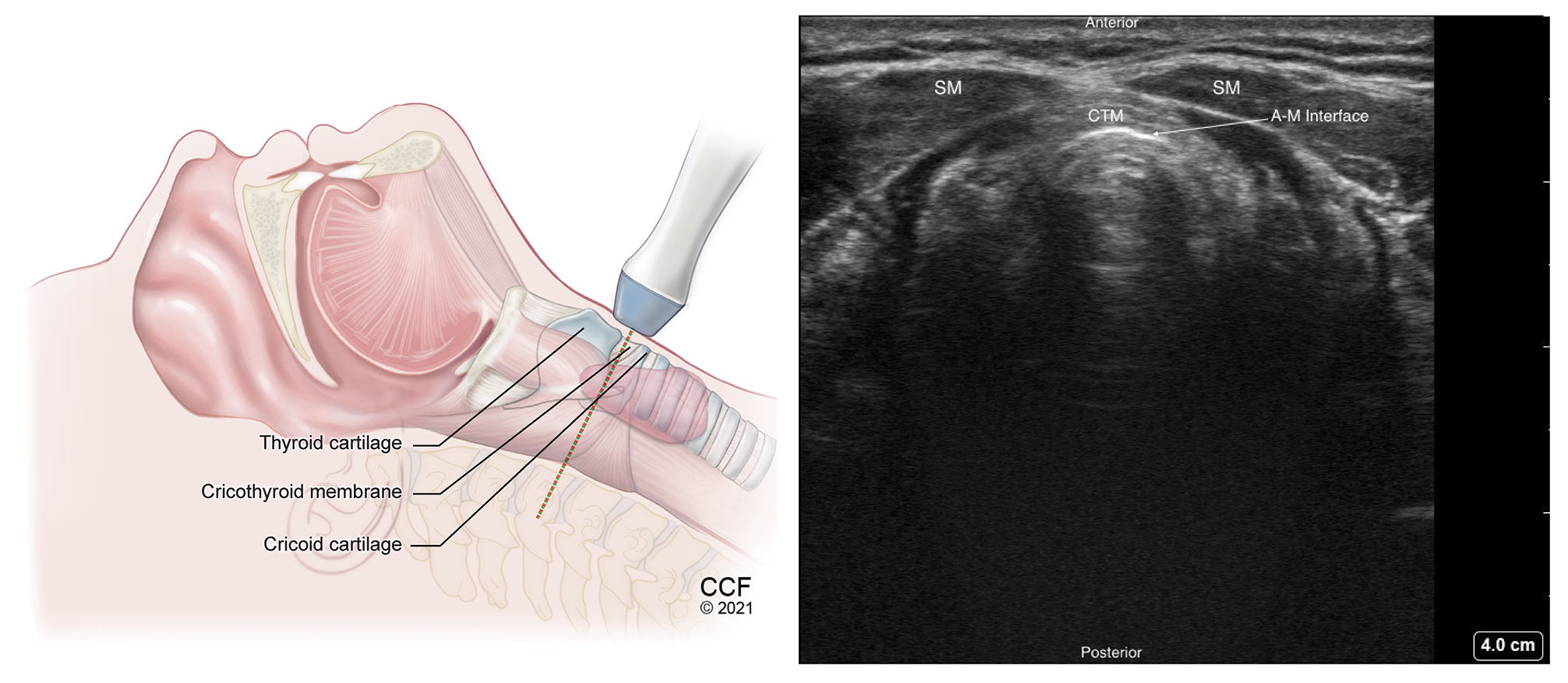 Figure 7. Transverse view of the cricothyroid membrane with high frequency linear transducer placed between the thyroid and cricoid cartilages.
Figure 7. Transverse view of the cricothyroid membrane with high frequency linear transducer placed between the thyroid and cricoid cartilages.
SM – Strap muscle, CTM – Cricothyroid membrane, A-M – Air-Mucosal Interface
Video 3. Cricothyroid Membrane Puncture
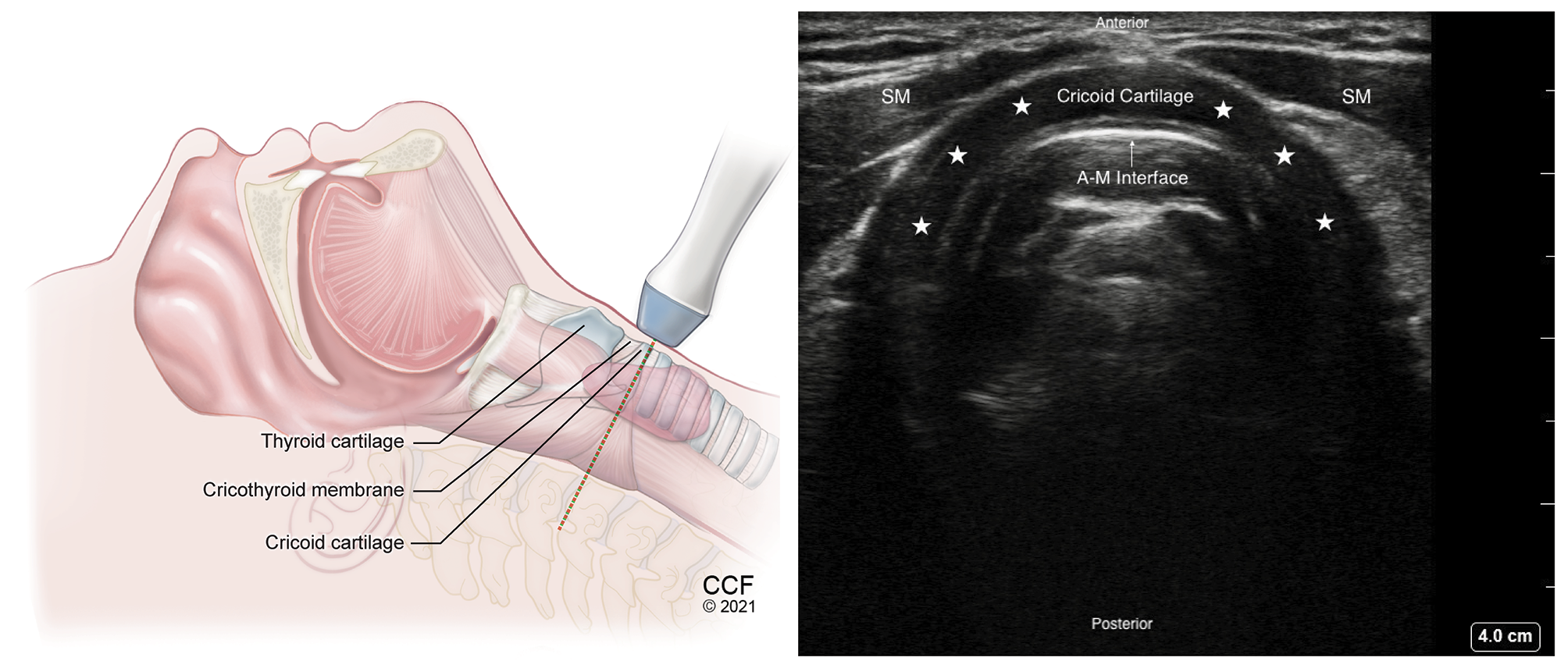
Figure 8. Transverse view of cricoid cartilage with high frequency linear transducer over the Cricoid cartilage. Hypoechoic arch shaped Cricoid cartilage marked with stars.
SM – Strap muscle, A-M – Air-Mucosal Interface
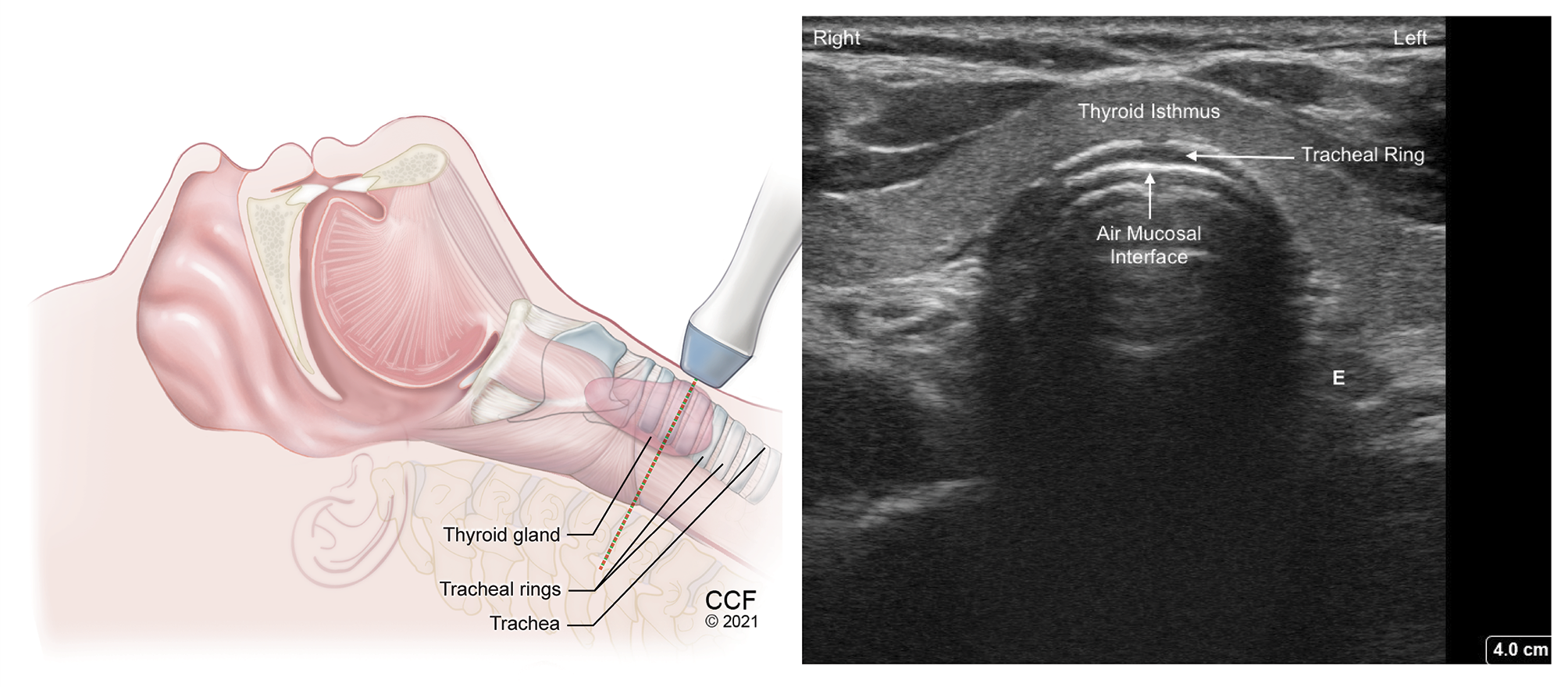
Figure 9. Transverse view of tracheal ring above suprasternal notch with high frequency linear transducer. Hypoechoic tracheal ring lined inside by hyperechoic A-M interface.
SM - Strap muscle, E - Esophagus to the left of trachea
Video 4. Detecting Esophageal Intubation
Identification of the Cricothyroid Membrane
The cricothyroid ligament, also called cricothyroid membrane (CTM), is composed of two components: one thick, median cricothyroid ligament and two lateral cricothyroid ligaments on each side, also called conus elasticus.
Digital palpation (DP) methods to identify the CTM are highly unreliable, and a recent systematic review concluded that ultrasound-guided CTM identification has enhanced accuracy, higher success, and lower complication rates compared to DP, especially
in patients with a difficult airway.2 The Difficult Airway Society (DAS) recommends that emergency front-of-neck access should be performed with the patient placed in the extended neck position.3 The sniffing position (head extension
and neck flexion) or neutral position have been shown to shift skin structures away from the true CTM in more than 50% of patients.4 Marking the mid-point of CTM prior to induction
of anesthesia should be done with the head and neck extended to prevent any displacement of the skin structures during airway manipulation.5
How I Do It
With the patient in the supine position, with the head and neck extension, place a high-frequency linear transducer at the level of thyroid cartilage in the transverse position. (Table 2) Identify the hypoechoic inverted V-shaped thyroid cartilage and
slide the transducer caudad to identify the CTM lined by hyperechoic air-mucosa (A-M) interface. Slide the transducer further caudally until the cricoid cartilage is identified as an arch-shaped hypoechoic structure with hyperechoic A-M interface
posteriorly. Having identified the cricoid, now move the transducer back cephalad to the mid cricothyroid membrane and mark it. This is called the TACA (Thyroid cartilage-Airline-Cricoid cartilage-Airline) technique, described by Kristensen et al.6 (Video
2) From this position the transducer can be rotated 90 degrees to get a longitudinal view of the thyroid and cricoid cartilages with the CTM in between.
The CTM also can be identified with the transducer placed longitudinally in the front of the neck above the suprasternal notch in the sagittal plane. The trachea can be identified by identification of hypoechoic tracheal rings resembling a “string-of-pearls” lined posteriorly by a bright A-M interface. With the transducer in a sagittal plane, slide in a cephalad direction, the cricoid cartilage can be identified as a larger and more superficial hypoechoic structure, compared to the tracheal rings. Further cephalad movement of the probe enables identification of the lower end of the thyroid cartilage in the cephalad position, the hyperechoic CTM in-between, and the cricoid cartilage caudally. Adjusting the transducer to bring the CTM to the center with one hand, use the other hand to slide a hyperechoic marker (metallic probe or a blunt needle) between the transducer and the patient’s skin from above. The marker is seen as hyperechoic dot with an acoustic shadow below, which can be adjusted to position midway between the inferior end of thyroid cartilage and the superior end of cricoid cartilage. At this point, the transducer is removed and the marker overlies the mid-point of CTM, where the cricothyroidotomy may be performed. (Video 3)
Table 2. I-AIM Framework for Airway Ultrasound 7
I = Indication | A = Acquisition of Image | I = Image Interpretation | M = Medical Decision-making |
Cricothyrotomy for elective/emergency airway access
Cricothyroid puncture for trans-tracheal topical anesthesia | Transverse scan sliding from thyroid cartilage to cricoid cartilage and back to CTM (TACA technique) Midsagittal scan over the thyroid and cricoid cartilages with hyperechoic CTM in between | Locating the level, mid-point, and size of the CTM (Video: 3) (Figure 10) | Determine safe and correct entry point for emergency airway access |
Determine safe and correct entry point for topicalization | |||
Confirmation of endotracheal intubation
Rule out esophageal intubation
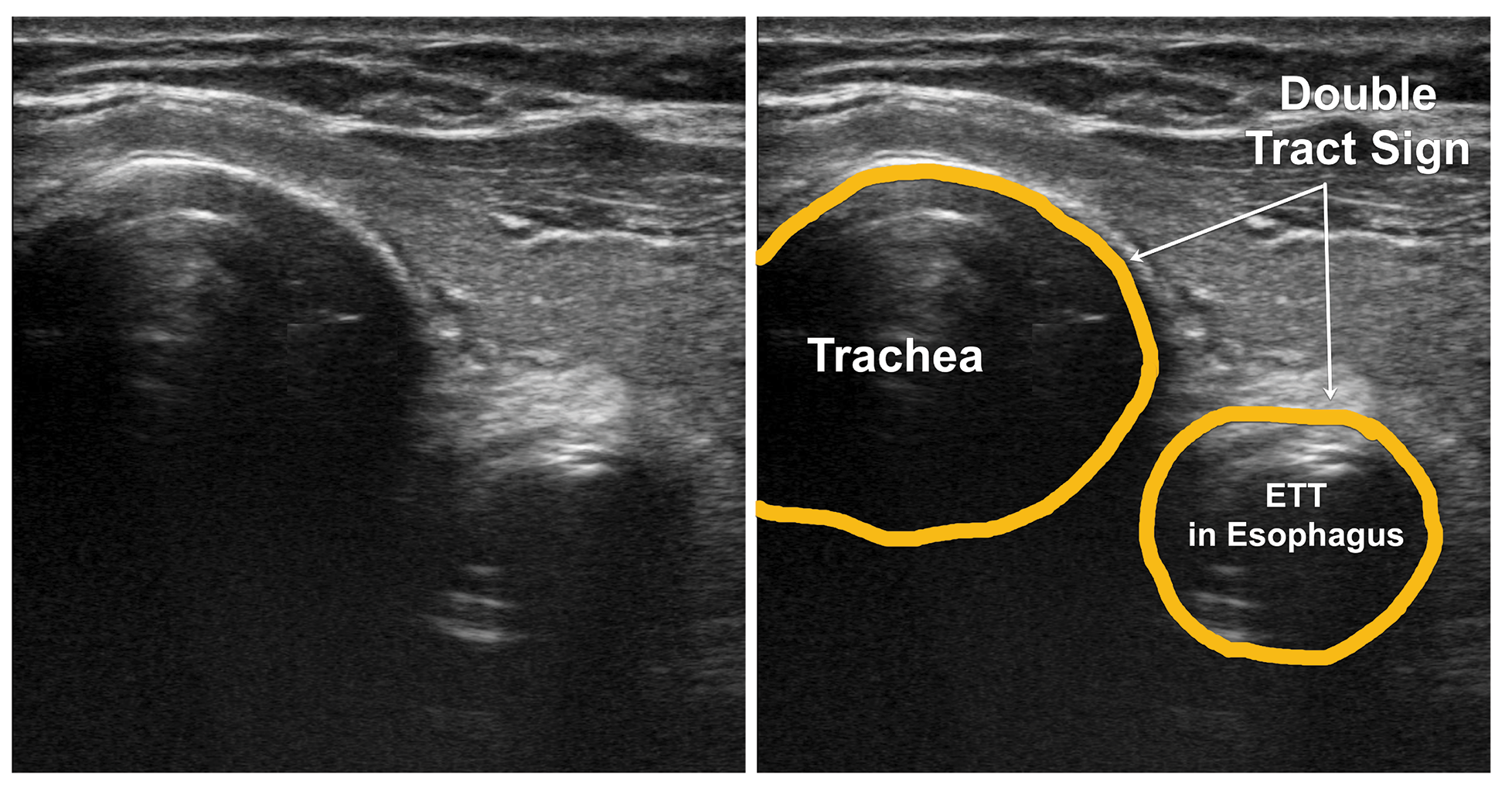
| Transverse scan over the glottis and/or trachea at suprasternal notch Scan both sides of chest for bilateral lung sliding | Movement of glottic structures as tube passes “Bullet” sign (Figure 13) for tracheal and “double tract” (Figure 14) sign for esophageal intubation (Video 4) | Determine the safety and use of the ETT for ventilation Reintubate if esophageal |
Superior laryngeal nerve block for awake fiberoptic intubation
| Parasagittal scan sliding laterally from midline over the front of neck | Identify the hyoid bone, thyroid cartilage, and hyperechoic thyrohyoid membrane between them (Figure 5) | Determine the feasibility of superior laryngeal nerve block; otherwise opt for landmark-guided technique |
Patient positioned supine with head and neck extended, use linear high frequency transducer.
CTM – cricothyroid membrane; ETT – endotracheal tube; TACA – (Thyroid Cartilage - Air line - Cricoid Cartilage - Airline)
Figure 10: Midsagittal scan of the neck with high frequency linear transducer over the suprasternal notch sliding up to thyroid cartilage.
Airway Blocks for Awake Fiberoptic Intubation
Awake fiber-optic intubation is the gold-standard technique in the management of a known difficult airway. Patient discomfort during the procedure can be mitigated by sedation and various techniques that achieve airway anesthesia. Topical application of local anesthetics by nebulization, spraying, and application at specific anatomical landmarks to block the afferent neural transmission from the oropharynx and larynx have been described.8 (Table 3) In the setting of the COVID-19 pandemic, it is important to prevent aerosol generation such as coughing or bucking during awake intubation.9 Techniques have been described to minimize aerosol generation during awake fiberoptic intubation, such as the use of a negative pressure tent, and may be used on a case-by-case basis.10 Nonetheless, ultrasound-guided airway blocks may supplement airway topicalization.
Table 3. Nerve Supply of the Airway
Nerve | Areas Supplied |
Trigeminal nerve (CN V) Anterior ethmoidal nerves (V1) Sphenopalatine nerves (V2) Palatine nerves (V2) Pharyngeal branch (V2) Lingual nerve (V3) |
Anterior nasal cavity |
Posterior nasal cavity | |
Hard and soft palate | |
Roof of the nasopharynx | |
Anterior 2/3rd of tongue | |
Glossopharyngeal nerve (CN IX) | Posterior 2/3rd of tongue, oropharynx, nasopharynx except roof, laryngopharynx, tonsils, pharyngeal surface of epiglottis |
Vagus nerve (CN X) Superior laryngeal nerve
Recurrent laryngeal nerve | Laryngeal surface of epiglottis and laryngeal structures above vocal cords including the superior surface of vocal folds |
Inferior surface of the vocal folds, larynx below the cords and trachea |
CN - cranial nerve, V1 - ophthalmic, V2 - maxillary, V3- mandibular branches of trigeminal nerve
To achieve complete airway anesthesia for orotracheal intubation, three types of regional blocks are needed: glossopharyngeal (oropharynx), superior laryngeal (larynx above the cords) and trans-laryngeal (larynx and trachea below the cords).8 (Figure 11)
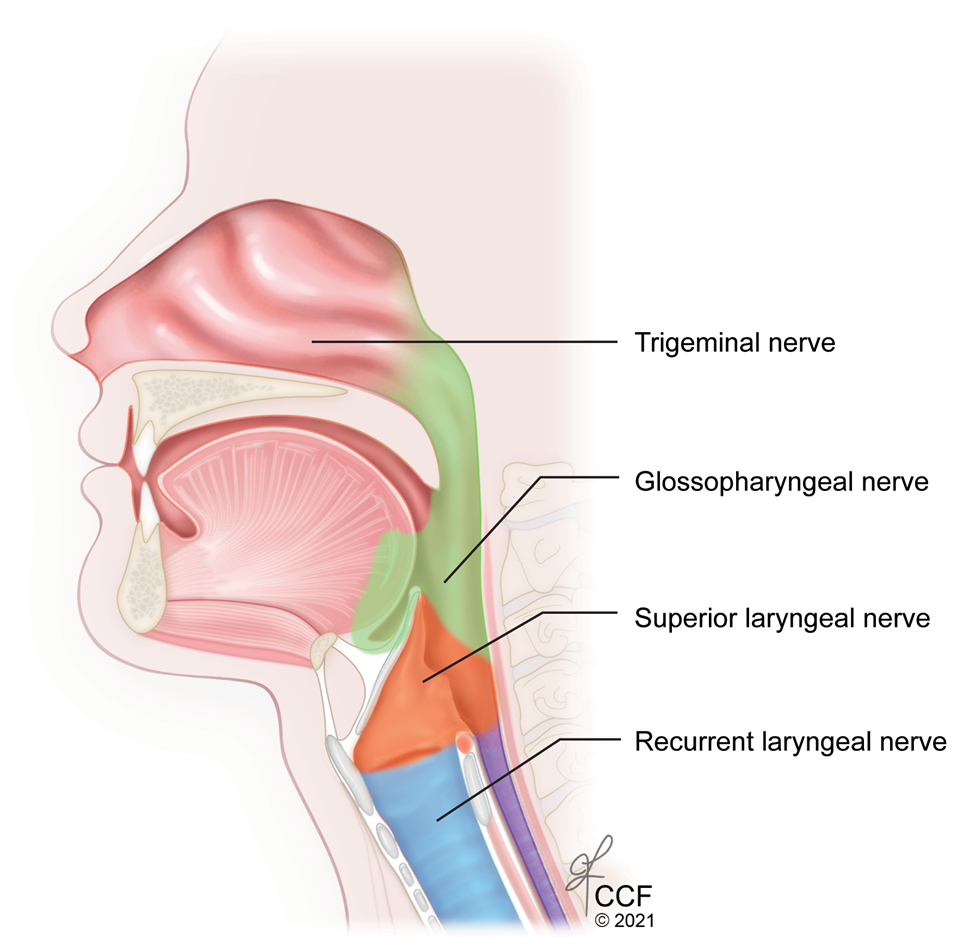
Figure 11: Sagittal section of the upper airway showing the sensory nerve supply
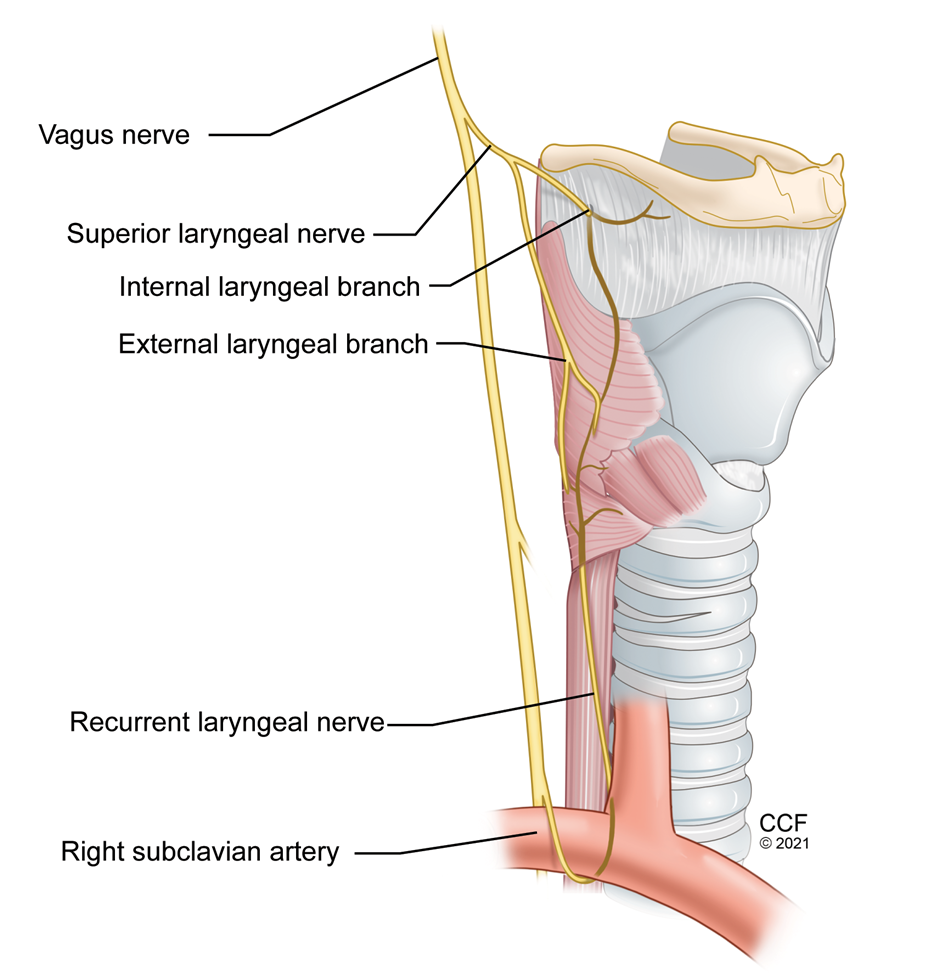
Figure 12: Nerve supply of Larynx
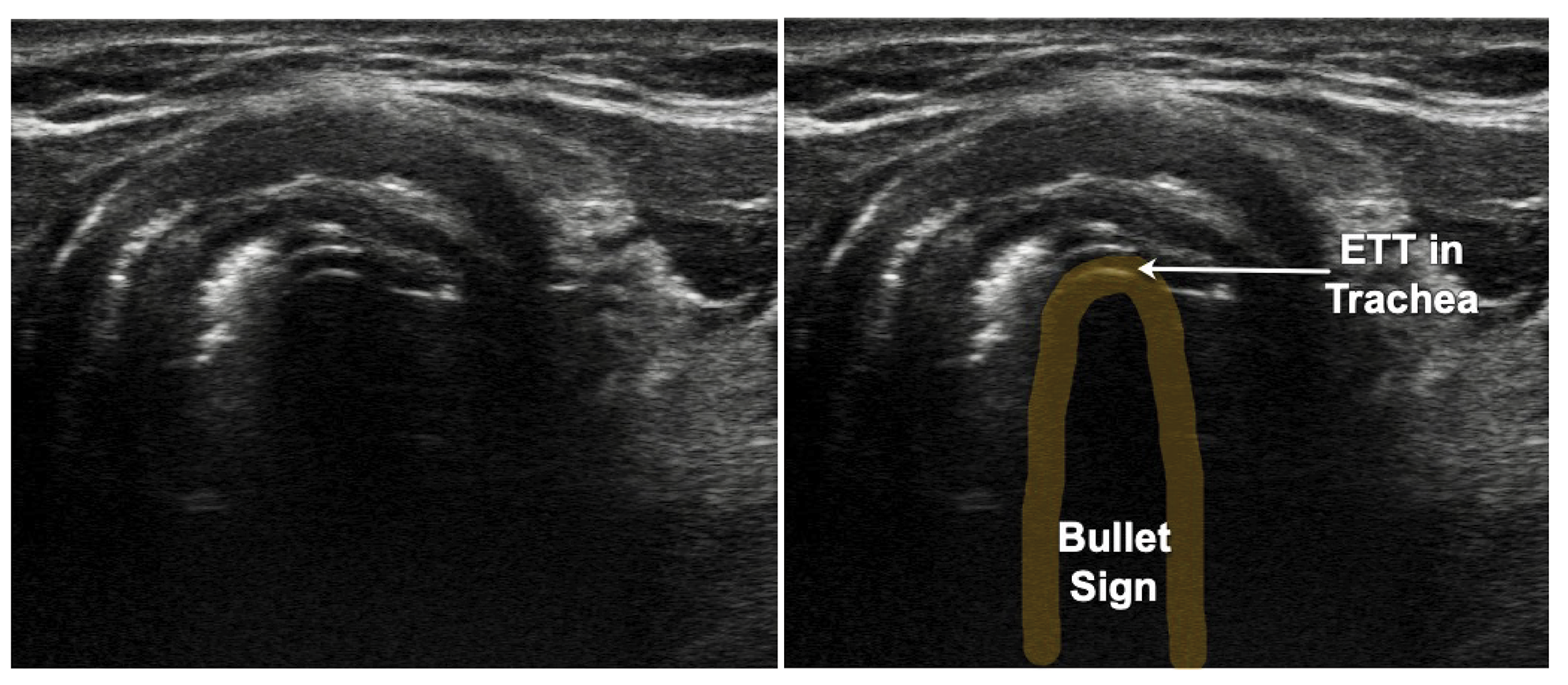
Figure 13: Endotracheal tube in the Trachea: Bullet Sign. ETT – Endotracheal tube
Figure 14: Transverse scan of the neck showing endotracheal tube in the esophagus. The double tract sign. ETT – Endotracheal tube
Glossopharyngeal Nerve Block
Ultrasound-guided glossopharyngeal nerve block has been described in the chronic pain practice to treat glossopharyngeal neuralgia.11 The nasal cavity, nasopharynx, oropharynx, and tongue can be easily anesthetized by topicalization without elicitation of coughing, gagging, or aerosol generation. Hence, we do not recommend specific glossopharyngeal and trigeminal nerve blocks for this purpose.
Superior Laryngeal Nerve Block
Superior laryngeal nerve is a branch of the vagus nerve. It divides into internal and external branches at the level of hyoid bone. The internal branch passes immediately inferior to the greater cornu of hyoid bone and enters through a foramen in the thyrohyoid membrane. (Figure 12) Sonographic visualization of the superior laryngeal nerve may not be easy and can be inconsistent even with locating the superior laryngeal artery. Hyoid bone, thyroid cartilage, and the thyrohyoid membrane are easy to identify with ultrasound, and depositing local anesthetic in the target tissue plane around the thyrohyoid membrane reliably blocks the internal branch of superior laryngeal nerve.12 (Figure 5)
How I Do It
As with any regional anesthetic technique, calculate toxic dose of the local anesthetic and prepare the room with appropriate monitors and local anesthetic systemic toxicity (LAST) protocol. With the patient in the supine position, with neck extended, the skin is prepped with chlorhexidine. Place a high-frequency linear transducer in the midline sagittal plane, and identify the hyoid bone (hyperechoic with a dropout shadow below), thyroid cartilage, and thyrohyoid membrane connecting the two. Slide the transducer laterally approximately midpoint between the anterior midline and the level of greater cornu of the hyoid bone postero-laterally. Insert a 22-gauge, 50-mm needle in-plane to puncture the thyrohyoid membrane and inject 2 ml of 2% lidocaine. Repeat on the other side. In patients with short necks, use transverse view or a transducer with a small footprint such as the hockey-stick probe.
Translaryngeal Injection
This block may induce coughing as the local anesthetic can initially irritate the tracheal mucosa; therefore, the patient should be warned, and the practitioner should consider using this injection to anesthetize the subglottic airway on a case-by-case basis. Identify the CTM, as described in the section above, in the sagittal plane. Slide a 22-gauge, IV cannula between the transducer and the patient’s neck from cranial end. (Video 3) Adjust the cannula by sliding and aligning it to the center of the CTM. Slowly withdraw the needle until it just disappears from the screen. At this point, remove the probe and tilt the needle upright, perpendicular to the skin, maintaining the entry point for the needle. (Video 3) Attach a 5-cc syringe with 2% lidocaine, and advance the 22-gauge IV cannula with gentle negative pressure until air is aspirated. Once trachea is entered, withdraw the needle and advance the sheath to prevent injury to the posterior wall of cricoid and trachea. Inject the local anesthetic to anesthetize the trachea, vocal cords, and larynx. The catheter can be left in place for later use, in case of an unable to intubate and ventilate scenario.
This block also can be performed under real-time ultrasound. After identifying the CTM in the sagittal plane in midline, the probe is moved slightly to a parasagittal position and tilted, keeping the CTM in view. The needle is inserted out-of-plane through the CTM, visualizing the needle tip as it enters the airway. Once air is aspirated, confirm the needle tip is in the airway and inject the local anesthetic.
Endotracheal Tube Confirmation
ETT confirmation with POCUS is highly accurate for both adult and pediatric patients.13,14 The accuracy remains consistent regardless of the ETT size or transducer type,14 with relatively short learning curve for using POCUS.15 ETT placement can be reliably detected during resuscitation with low cardiac output and low end-tidal carbon dioxide.16 The transducer is placed in transverse position in the suprasternal notch. Observe for movement in the trachea appearing like a snowstorm as the ETT passes across the probe, called the “snowstorm sign.” If the ETT enters the esophagus, the unique “double tract sign” is seen.17 (Video 4) If only one operator is present, postintubation check with twisting the ETT side to side, using the color doppler while moving improves the diagnostic accuracy. Inflation of the ETT cuff with saline also may improve the ability to identify and confirm ETT location. Adding the lung-sliding sign to this method improves diagnostic accuracy of endotracheal intubation.
Other Clinical Uses
POCUS for upper airway has been described to assess and predict difficult mask ventilation and difficult laryngoscopy using skin-hyoid distance,18 while the epiglottis to vocal cord space has been shown to correlate with Cormack-Lehane intubation grade. It also has been studied for screening obstructive sleep apnea19 and evaluation of vocal cords and epiglottis.20 Airway ultrasound is utilized for identification of tracheal rings for correct location and safe placement of percutaneous tracheostomy avoiding pre-tracheal blood vessels in both elective and emergency situations.21,22
Conclusion
POCUS airway is a non-invasive, reliable, and relatively simple technique that is reproducible to obtain real-time dynamic images of the upper airway anatomy. Application of POCUS airway has been useful to perform elective and emergency procedures such as confirmation of endotracheal intubation and cricothyrotomy, as well as facilitating upper airway regional techniques. Further research and innovation are under way to examine other potential uses such as assessment of difficult laryngoscopy or screening for risk of obstructive sleep apnea.

Sree Kolli, MD, EDRA, is a clinical assistant professor in the department of General Anesthesiology and Pain Management at the Anesthesiology Institute of Cleveland Clinic in Cleveland, OH.
Mandeep Singh, MBBS, MD, MSc, FRCPC, is an assistant professor in the department of Anesthesiology and Pain Management at Women’s College Hospital, University Health Network, and University of Toronto in Toronto, Canada.
References
- Singh M, Chin KJ, Chan VWS, Wong DT, Prasad GA, Yu E. Use of sonography for airway assessment. J Ultrasound Med 2010;29(1):79-85. https://doi.org/10.7863/jum.2010.29.1.79.
- Rai Y, You-Ten E, Zasso F, De Castro C, Ye XY, Siddiqui N. The role of ultrasound in front-of-neck access for cricothyroid membrane identification: A systematic review. J Crit Care 2020;60:161-168. https://doi.org/10.1016/j.jcrc.2020.07.030.
- Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 2015;115(6):827-48. https://doi.org/10.1093/bja/aev371.
- Dixit A, Ramaswamy KK, Perera S, Sukumar V, Frerk C. Impact of change in head and neck position on ultrasound localisation of the cricothyroid membrane: an observational study. Anaesthesia 2019;74(1):29-32. https://doi.org/10.1111/anae.14445.
- Bowness J, Teoh WH, Kristensen MS, Dalton A, Saint-Grant AL, Taylor A, et al. A marking of the cricothyroid membrane with extended neck returns to correct position after neck manipulation and repositioning. Acta Anaesthesiol Scand. 2020;64(10):1422-1425. https://doi.org/10.1111/aas.13680.
- Kristensen MS, Teoh WH, Rudolph SS, Tvede MF, Hesselfeldt R, Borglum J, et al. Structured approach to ultrasound-guided identification of the cricothyroid membrane: A randomized comparison with the palpation method in the morbidly obese. Br J Anaesth 2015;114:1003-4. https://doi.org/10.1093/bja/aev123.
- Haskins SC, Bronshteyn Y, Perlas A, El-Boghdadly K, Zimmerman J, Silva M, et al. American Society of Regional Anesthesia and Pain Medicine expert panel recommendations on point-of-care ultrasound education and training for regional anesthesiologists and pain physicians - part I: clinical indications. Reg Anesth Pain Med. Published online first 2021, February 24:1-17. https://doi.org/10.1136/rapm-2021-102560.
- Simmons ST, Schleich AR. Airway regional anesthesia for awake fiberoptic intubation. Reg Anesth Pain Med 2002;27(2):180-92. https://doi.org/10.1053/rapm.2002.30659.
- Cergan R, Dumitru M, Vrinceanu D, Neagos A, Jeican I, Ciuluvica R. Ultrasonography of the larynx: novel use during the SARS‑CoV‑2 pandemic (Review). Exp Ther Med 2021;21(3):1-5. https://doi.org/10.3892/etm.2021.9704.
- Ip V, Tham C. COVID-19 pandemic: negative-pressure tent during atomization of local anesthetic for awake fiberoptic intubation. Anesth Analg 2020;131(3):e178-e179. https://doi.org/10.1213/ANE.0000000000005066.
- Azman J, Pintaric TS, Cvetko E, Vlassakov K. Ultrasound-guided glossopharyngeal nerve block a cadaver and a volunteer sonoanatomy study. Reg Anesth Pain Med 2017;42(2):252-8. https://doi.org/10.1097/AAP.0000000000000561.
- Stopar-Pintaric T, Vlassakov K, Azman J, Cvetko E. The thyrohyoid membrane as a target for ultrasonography-guided block of the internal branch of the superior laryngeal nerve. J Clin Anesth 2015;27(7):548-52. https://doi.org/10.1016/j.jclinane.2015.07.016.
- Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta-analysis. Can J Anesth 2015;62(4):413-423. https://doi.org/10.1007/s12630-014-0301-z.
- Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta-analysis. Ann Emerg Med 2018;72(6):627-36. https://doi.org/10.1016/j.annemergmed.2018.06.024.
- Chenkin J, McCartney CJL, Jelic T, Romano M, Heslop C, Bandiera G. Defining the learning curve of point-of-care ultrasound for confirming endotracheal tube placement by emergency physicians. Crit Ultrasound J 2015;7(1). https://doi.org/10.1186/s13089-015-0031-7.
- Chou H-C, Chong K-M, Sim S-S, Ma MH-M, Liu S-H, Chen N-C, Wu M-C, et al. Real-time tracheal ultrasonography for confirmation of endotracheal tube placement during cardiopulmonary resuscitation. Resuscitation 2013;84(12):1708-12. https://doi.org/10.1016/j.resuscitation.2013.06.018.
- Chou H-C, Tseng W-P, Wang C-H, Ma MH-M, Wang H-P, Huang P-C, et al. Tracheal rapid ultrasound exam (T.R.U.E.) for confirming endotracheal tube placement during emergency intubation. Resuscitation 2011;82(10):1279-84. https://doi.org/10.1016/j.resuscitation.2011.05.016.
- Alessandri F, Antenucci G, Piervincenzi E, Buonopane C, Bellucci R, Andreoli C, et al. Ultrasound as a new tool in the assessment of airway difficulties: an observational study. Eur J Anaesthesiol 2019;36(7):509-15. https://doi.org/10.1097/EJA.0000000000000989.
- Singh M, Tuteja A, Wong DT, Goel A, Trivedi A, Tomlinson G, et al. Point-of-care ultrasound for obstructive sleep apnea screening: are we there yet? a systematic review and meta-analysis. Anesth Analg 2019;129(6):1673-91. https://doi.org/10.1213/ANE.0000000000004350.
- Ko DR, Chung YE, Park I, Lee H-J, Park JW, You JS, et al. Use of bedside sonography for diagnosing acute epiglottitis in the emergency department. J Ultrasound Med 2012;31(1):19-22. https://doi.org/10.7863/jum.2012.31.1.19.
- Alansari M, Alotair H, Al Aseri Z, Elhoseny MA. Use of ultrasound guidance to improve the safety of percutaneous dilatational tracheostomy: a literature review. Crit Care 2015;19(1)229. https://doi.org/10.1186/s13054-015-0942-5.
- Ravi PR, Vijai MN, Shouche S. Realtime ultrasound guided percutaneous tracheostomy in emergency setting: the glass ceiling has been broken. Disaster Mil Med. 2017;3(1):7-12. https://doi.org/10.1186/s40696-017-0035-x.

Leave a commentOrder by
Newest on top Oldest on top