Chronic Pain in Children and Adolescents
Ravi Shah, MD
Santhanam Suresh, MD, FAAP
INTRODUCTION
Chronic pain is a significant and underreported problem in the pediatric population that carries psychological, emotional, and social repercussions for both the child and family.1,2 The potential for such consequences to negatively impact a child’s quality of life has fostered the development of a multidisciplinary approach to treat pediatric pain.3, 4 A variety of behavioral, pharmacological, and physical therapies are employed in pediatric chronic pain treatment regimens. Interventional procedures may be introduced after patients fail other treatment approaches.5
Chronic pain in childhood and adolescence is more common than what is reported and prevalence rates vary substantially between data from different studies.6 Children experiencing persistent or recurrent chronic pain may miss school or withdraw from social activities, and are at risk of developing internalizing symptoms in response to their pain. Given these consequences, researchers and clinicians are working to develop effective multidisciplinary strategies to ameliorate chronic pain in children and adolescents.
ASSESSMENT OF CHRONIC PAIN IN CHILDREN
Assessment of children with chronic pain requires a biopsychosocial perspective to take into account the various factors that influence a child’s pain experience. Multidimensional models elaborate various biologic, developmental, temperamental, cognitive-behavioral, affective, social, and situational factors that shape the child’s pain experience.7,8 Each domain may become a target of assessment and intervention. Several developmentally sensitive validated instruments are now available to measure the varied aspects of a child’s pain. (Table 1)
Table 1. Methods for assessment of chronic pain in children and adolescents
Pain Measures | Disability or Quality of Life Assessment Tools | Other Behavioral Measures |
Varni-Thompson Pediatric Pain Questionnaire (Ages 5-18 years) | Functional Disability Inventory (Ages 8-17 years) | Children’s Somatization Inventory (Ages 8-18 years) |
Children’s Comprehensive Pain Questionnaire (Ages 5-19 years) | Child Health Questionnaire (Ages 5+) | Harter Scales of Perceived Competence for Children (Ages 4-12) |
Pain Behavior Observation Method (Ages 6-17) | Children’s Activity Limitations Scale (Ages 8-16 years) | |
Pain Diary (Ages 8+) |
The Children’s Comprehensive Pain Questionnaire (CCPQ)9 and the Varni-Thompson Pediatric Pain Questionnaire (VTPPQ)10 are age-specific standardized interviews for school-age and adolescent children and their parents that provide comprehensive evaluations of a child’s chronic pain. Both interviews separately assess the child’s and parents’ experience of the pain-related problems with open-ended questions, checklists, and quantitative pain-rating scales. Some studies suggest potential limitations of these self-report measures because of cultural or cognitive differences among families.11 The Pain Behavior Observation Method is a 10-minute observational pain behavior measure that can be used in children who may have difficulty with self-report measures because of age-related or cognitive limitations.12 Electronic diary assessment of pain and disability has gained popularity in recent years and studies have supported their use in children with chronic pain, demonstrating increased compliance and accuracy in diary recording when compared to traditional paper diaries.13
The ability to function the tasks of daily living is an important outcome measure to assess when treating children and adolescents with chronic pain. In some cases, pain cannot be completely relieved and the child must learn to cope and adapt to the pain in order to participate in normal developmental activities and tasks, such as attending school, participating in extracurricular activities, and maintaining social relationships. Various measures have been developed to assess the child’s functional ability. The Functional Disability Inventory (FDI) was developed to assess illness-related disability in children and adolescents. It is particularly useful for children with pain disorders that are associated with psychological factors and pain-associated disability.14-16 The Child Health Questionnaire may be used to assess general quality of life in children with chronic pain and has the advantage that the scores obtained can be compared with standardized samples of scores obtained by children with other medical illnesses. The Child Activity Limitations Interview (CALI) measures the impact of recurrent pain on the child’s daily activities as a way to identify appropriate targets for treatment.17
Other instruments used to evaluate psychological factors that are contributing to a child’s behavioral adaptation to chronic pain include the Children’s Somatization Inventory (CSI),18 which measures a child’s propensity towards somatization, and the Harter Scales of Perceived Competence,19 which assess a child’s judgment about his or her capabilities in functional domains such as school performance, peer relationships, and athletic abilities.
MULTIDISCIPLINARY APPROACH TO PEDIATRIC CHRONIC PAIN MANAGEMENT
The introduction of multidisciplinary chronic pain management programs has allowed children to be evaluated and treated by a number of consultants during a single office visit. Many pediatric pain clinics are composed of an anesthesiologist specialized in pain management, a child psychologist with a special interest in pain, physical therapists, complementary medicine including massage therapy and acupuncture therapy, as well as biofeedback. This comprehensive approach enables patients to receive better care with minimal disruption to their lives.
Psychological pain management methods are aimed toward increasing the child’s and family’s understanding of the child’s pain and its treatment, focusing on factors that may reduce or exacerbate the child’s pain. The child’s cognitive and behavioral coping skills are fostered in an effort to reduce pain-related discomfort and disability. In a meta-analysis to evaluate the efficacy of behavioral interventions for pediatric chronic pain, Eccleston and collegues concluded that strong evidence exists to support psychological treatments, specifically relaxation and cognitive behavioral therapy, as effective methods to reduce the severity and frequency of chronic pain in children and adolescents. 20 Other studies have suggested that interdisciplinary pediatric pain rehabilitation may facilitate increased willingness to self-manage pain, which is associated with improvements in function and psychological well-being. 21
Physical therapy is geared toward reestablishing adequate functional ability of the child. In children, physical therapy is especially useful in cases of myofascial pain and can be implemented at a rehabiliation facility, home or school.22 In younger children, these exercises can take the form of play that is geared toward improving musculoskeletal function, fine and gross motor function, posture, endurance and circulation. Restoring the patient’s physical ability can help the child participate in activities of daily living and improve overall function.
Complementary and alternative medicine (CAM) is defined by the National Center for Complementary and Alternative Medicine as “a group of diverse medical and health care systems, practices, and products that are not generally considered to be part of conventional medicine.”23 Biofeedback, hypnosis, guided-imagery, mindfulness, massage, and acupuncture have been used as adjuncts to the treatment of chronic, acute, and recurrent pain for both pediatric and adult populations.24-27 A systematic review suggested that gut-directed hypnotherapy for functional abdominal pain and irritable bowel syndrome was considered superior to traditional medical management of such conditions. Although limited data are available to support the efficacy of CAM in pediatric patients, such treatments offer the potential for pain relief with a relatively low incidence of side effects.28
Many tertiary pain centers in the United States have adopted a multidisciplinary approach to pain management that incorporates use of at least a subset of CAM modalities. In a 2005 survey of 43 pediatric anesthesiology fellowship programs, 38 reported that their clinical services to patients included at least one CAM modality, including biofeedback (65%), guided imagery (49%), relaxation therapy (33%), massage (35%), hypnosis (44%), acupuncture (33%), art therapy (21%), and meditation (21%).29 Taso et. al. have suggested that the longer a child experiences pain, the more likely he or she is to express an interest in trying CAM treatment approaches.30
PEDIATRIC CHRONIC PAIN SYNDROMES
The following sections will discuss the diagnosis and management of some common chronic pain syndromes diagnosed in pediatric patients referred to chronic pain clinics, including Complex Regional Pain Syndrome Type I, headache, abdominal pain, and cancer pain.
COMPLEX REGIONAL PAIN SYNDROME TYPE I
Complex regional pain syndrome type I (CRPS I) presents with a group of symptoms that consist of pain, allodynia, hyperalgesia, and possible loss of function. The International Association for the Study of Pain (IASP) has defined CRPS I as “a continuous pain in a portion of an extremity after trauma, which may include fracture but does not involve major nerve lesions and is associated with sympathetic hyperactivity.” 31 In pediatric patients, CRPS I more commonly occurs in the lower extremity and the majority of children involved are female, many of whom have endured minor trauma before the development of chronic pain.32-34 Although it has been reported in a child as young as 2.5 years old, it is generally seen in children older than 9 years and more frequently in girls 11 to 13 years of age. 35,36 Early recognition and management are the major factors in improving outcome and preventing resistant CRPS.37 Management should include a multidisciplinary approach. Psychological evaluation and cognitive-behavioral treatment should be provided in an expeditious manner.
EVALUATION
A detailed history of the nature of the injury that includes the type and duration of pain, relieving and aggravating factors, and dependence on medications should be performed prior to physical examination. A thorough and systematic neurologic examination should be performed with evaluation of motor, sensory, cerebellar, cranial nerve, reflex, cognitive, and emotional functioning. A concerted effort should be made to rule out a rare, but possible malignancy or central degenerative disorder. Allodynia is a common finding and hyperalgesia to cold is seen more frequently than sensitivity to heat.38 In children, the distribution is not generally restricted to particular dermatomes and commonly occurs along a glove-and-stocking distribution. Nerve conduction studies may provide insight into the nature of a nerve injury, however, the use of invasive electromyography may not be acceptable to children.39 Quantitative sensory testing (QST) in the affected limbs can be compared with data from normal healthy children. Although this involves cumbersome equipment, bedside QST may play a role in the diagnosis of CRPS I in children and adolescents.40 Bone scans may be helpful in the diagnosis of CRPS I. Although insufficient data exist to support their diagnostic accuracy in children, they can nevertheless be performed in children and adolescents with suspected CRPS I.41
TREATMENT
Management of CRPS I can be frustrating for both the caregiver and the patient as no single therapy can uniformly provide relief of symptoms. Titration of medications is limited by the presence of side effects and complications. It is imperative to return the child to a functional state, including attendance at school. Most CRPS I management techniques that are used in children have been extrapolated from the adult literature.42
Behavioral measures are extremely useful in the management of CRPS I in children and adolescents. Group therapy often helps family members cope with the situation.43 We generally advocate consultation with a medical psychologist during the initial visit to the pain clinic. Several techniques, including biofeedback, visual guided imagery, and structured counseling, have been shown to assist in the development of adequate coping skills.44 Participation in a day program for acute psychological intervention has been valuable for some of our patients, specifically those with significant psychiatric co-illness.
Physical therapy is geared toward restoring adequate functional ability of the child. Transcutaneous electrical nerve stimulation (TENS) is widely used, and its efficacy has been studied in adults as well as children; therapeutic benefits with TENS in children with CRPS I have been reported by Kesler and colleagues.45 We use TENS extensively in our practice, along with physical therapy, which consists of both active and passive physical modalities. The physical therapy program is geared toward individual patients, and the goal is to allow the child to participate in as many activities as possible. Other commonly used modalities include desensitization, warm and cold baths, massage therapy, and heat therapy. Such modalities, when used in conjunction with active physical modalities, can help ameliorate pain symptoms.46
Therapeutic adjuncts used to treat pediatric CRPS I, including pharmacotherapy, regional anesthesia and sympathetic blockade. Most treatment approaches in extrapolated from efficacy data in adults.
Tricyclic Antidepressants
Despite the lack of adequately controlled studies in pediatric patients, tricyclic antidepressants (TCAs) are widely prescribed for several forms of neuropathic pain.47 Because amitriptyline may cause sedation and other anticholinergic side effects, nortriptyline is often used as an alternative in children. Thorough examination of the cardiovascular system is necessary before instituting TCA treatment because of associated tachydysrhythmia and other conduction abnormalities of the heart, particularly prolonged QT syndrome.48,49
Anticonvulsants
Anticonvulsant medications are commonly used to manage neuropathic pain in pediatric patients, especially since the introduction of gabapentin and pregabalin. Despite the lack of controlled trials in children to demonstrate the efficacy of either drug, both of these medications have been used with promising results.50 More controlled trials should be conducted to better determine the dosing and efficacy of this class of drugs in children with CRPS I. An important side effect that we have noted in our clinic setting is the potential for increased somnolence, as well as the potential for weight gain in children taking pregabalin. This is important to consider, especially when treating adolescent girls, who happen to be the majority of this cohort.
Selective Serotonin Reuptake Inhibitors and Serotonin-Norepinephrine Reuptake Inhibitors.
Despite the lack of proven efficacy of the use of selective serotonin reuptake inhibitors in the management of pain in children and adolescents, they are occasionally used to treat psychological comorbidity, including pain-associated depression.51 More recently, serotonin-norepinephrine reuptake inhibitors have been introduced and used successfully to treat neuropathic pain, especially in patients with psychological comorbidity.52
Systemic Vasodilators
Several patients with CRPS I have benefited from the use of vasodilators such as prazosin, nifedipine, and phenoxybenzamine. However, the adverse effects of orthostatic hypotension often offset the efficacy of this therapy.
Regional Anesthesia and Sympathetic Blocks
A common treatment of these syndromes is to interrupt the apparent pathologic reflexes by performing sympathetic blocks. Regional anesthesia, which is often utilized in adults for the diagnosis and management of CRPS I, is generally introduced in children after pharmacological and cognitive-behavioral management have been exhausted. In severe cases, regional anesthesia is used to introduce a physical therapy regimen.
Central neuraxial blockade may be performed in children with severe pain to facilitate the introduction of physical therapy. Intrathecal analgesia has been reported to be an effective method for treating refractory CRPS I in children.53 Bier block has been used for mild to moderate cases of CRPS I as a primary modality for providing analgesia and sympathetic blockade.54 Although various substances have been used to provide a Bier block, a local anesthetic in combination with either a α2-agonist or an NSAID appears to produce better results.55
Peripheral nerve blocks are used to facilitate physical therapy while providing a sympathectomy and have become more feasible, especially with the use of ultrasound guidance.5 Serial peripheral nerve blocks are often performed, after which the patient’s pain relief may outlast the duration of conduction blockade. Continuous peripheral nerve blocks (CPNBs) have been reported to be effective in both controlling pain and facilitating physical therapy in children with CRPS.56 Despite such reports, limited data exist regarding the feasibility, safety, and efficacy of CPNBs for the treatment of CRPS I in children.57
Sympathetic blockade is used in children after exhausting the aforementioned techniques. A crossover trial of fluoroscopically-guided lumbar sympathetic blocks demonstrated a decrease in allodynia and pain intensity when compared with intravenous injection of lidocaine in adolescents with CRPS.58 Neuromodulation via spinal cord stimulation, though commonly performed in adults for refractory cases of CRPS, is very rarely used in the pediatric setting.59,60 Spinal cord stimulation has been reported to achieve favorable outcomes in adolescents with therapy-resistant CRPS.61
Ashwal and associates concluded that the prognosis of childhood CRPS I is more favorable than that of adult CRPS I.62 Neuropathic pain can be puzzling and frustrating and requires a strong alliance with the family and the patient. A multidisciplinary algorithmic management approach can be helpful. The use of physical therapy and psychological management must be stressed while managing these patients.
HEADACHES IN CHILDREN
Headache is a common neurological symptom reported in childhood and adolescence and is associated with several comorbid conditions, particularly with respect to the neurological, psychiatric and cardiovascular systems. Symptoms in children can cause significant impairment, leading to school absences and withdrawal from daily activities.
Bille et al reported migraine headaches to occur in 3.9% of children younger than 12 years and noted a 6.8% incidence of nonmigrainous headaches daily.63 Childhood headaches are commonly associated with psychiatric and neurological comorbidity, in particular depression and anxiety, epilepsy, sleep disorders, and ADHD.64 Association with cardiovascular disease, especially ischemic stroke and PFO, has also been reported in the pediatric population.65
EVALUATION AND MANAGEMENT OF HEADACHE
A thorough history and physical examination should be performed to determine the nature of the headache. Neurologic symptoms such as ataxia, lethargy, seizures, or visual impairment should be noted and other medical conditions such as hypertension, sinusitis, and emotional disturbances must be evaluated. Physical examination should include a thorough neurologic examination and blood pressure measurement. Neuroimaging may be required and a lumbar puncture might be advised in some cases. Benign intracranial hypertension or idiopathic intracranial hypertension is a constellation of symptoms that includes headaches, diplopia, tinnitus, and eye pain in the presence of normal imaging results.66
Childhood headaches are associated with comorbid symptoms. Sleep deprivation is a common finding and delayed sleep is a frequent disorder seen in children with headaches. Many children with headache also report dizziness, which can be associated with postural hypotension and tachycardia (postural orthostatic tachycardia syndrome).67 A history of a new-onset severe headache, pain that awakens a child from sleep, headaches associated with straining, or the presence of a headache accompanied by nausea or vomiting suggests a more pathologic origin of the headache and must be carefully evaluated.
After careful evaluation and classification of the type of headache, treatment is initiated in stepwise fashion. We use an algorithm [Figure 1] for the management of headaches. Tension-type headache is perhaps the most common type of headache that we see in our pain clinic. These patients commonly complain of debilitating frontotemporal or frontoparietal headaches, often due to contraction of the temporalis muscle and tension on the scalp muscles.68 Management of tension-type headaches includes the use of relaxation techniques, as well as biofeedback. These patients frequently benefit from the routine use of nonsteroidal agents.69 In addition, caffeine has been described as an effective adjuvant to nonsteroidal drugs in treating childhood headaches.70
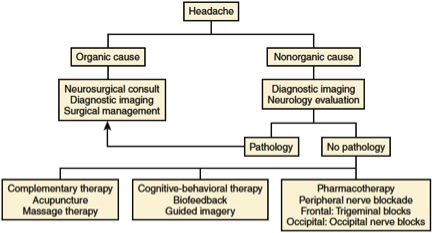
Figure 1. Algorithm for management of headache in children
Adapted from Suresh S, Shah R: Chronic Pain Management in Children and Adolescents. In Benzon, Raja, Liu, Fishman, Cohen: Essentials of Pain Medicine, 3rd ed. Philadelphia, Elsevier Saunders, 2011, 406.
Children occasionally suffer from persistent neuropathic headaches. This commonly occurs in those who have undergone ventriculoperitoneal shunt revision or surgical decompression for a Chiari malformation. After first utilizing cognitive-behavioral therapy and pharmacotherapy, we attempt to use serial peripheral nerve blocks in these patients. This includes trigeminal nerve blocks for frontal headaches and occipital nerve blocks for occipital headaches. An ultrasound-guided approach to the occipital nerve allows easy access to the C2 nerve root, thereby providing a more robust blockade than can be achieved with a peripheral subcutaneous injection.71
ABDOMINAL PAIN IN CHILDREN
Abdominal pain is commonly encountered in the pediatric population. Functional abdominal pain (FAP) is considered to be pain unrelated to an identifiable organic gastrointestinal disorder.72 Once a diagnosis of FAP is established, cognitive-behavioral therapy combined with family-centered therapy can be an treatment strategy.73 Several authors have described an affective component of FAP.74 Walker and associates suggested that children with FAP are at increased risk for the development of chronic pain in adulthood.75 This is potentially due to mechanisms linked to heightened central sensitization.76 Amitriptyline has been described as an effective treatment of FAP in children, although a prospective randomized controlled trial demonstrated no significant difference between placebo and amitriptyline.77
We demonstrated the efficacy of serial nerve blocks in children with chronic abdominal pain, particularly those in whom neuropathic pain develops after abdominal surgery. Specifically, ultrasound-guided rectus sheath blocks or transversus abdominis plane blocks resulted in decreased pain scores in our cohort.78 Ilioinguinal neuralgia following hernia repair is an under-reported cause of abdominal pain in older children and adolescents.79 Persistent pain likely results from major dissection during surgery. TENS may be helpful and peripheral nerve blocks can be used to manage pain. Serial ultrasound-guided ilioinguinal nerve blocks have been effectively utilized as a treatment modality in this setting.80
PEDIATRIC CANCER PAIN
Cancer is diagnosed in more than 12,000 children annually, and approximately 2200 children die each year of this disease.81 The incidence of cancer-related pain in children at the time of diagnosis is estimated to be 75 percent, with ongoing pain affecting 50 percent of patients.82, 83 During the terminal phase of disease, the incidence of pain is approaches 90 percent.84 Cancer pain in children is can be related to several etiologies: (1) tumor-related pain (e.g., solid tumor or bony metastatic tumors), (2) pain caused by treatment (e.g., mucositis or surgical pain), and (3) neuropathic pain secondary to tumor invasion or surgery. Pain caused by either a treatment or procedure is cited as the most frequent type of pain experienced by children with cancer.85 Management of pediatric cancer-related pain must be individualized, and caregivers must be empathetic to family needs and concerns. Although most pain complaints can be managed by implementation of the World Health Organization (WHO) cancer pain ladder paradigm, a significant number of children may require additional therapies or techniques for pain management because of escalating or intractable pain.
Although systemic analgesic therapies are the mainstay of pain treatment in pediatric palliative care, there are cases where they fail to adequately relieve symptoms or produce side effects that undermine effectiveness. Regional anesthesia or other procedural interventions may be considered as a potential therapy for these patients. 86 However, the use of such techniques in children is supported only by case reports, case series, and very few randomized controlled studies.
CONCLUSIONS
Chronic pain in children remains an under-recognized entity. Early diagnosis and intervention are helpful in achieving adequate recovery. A multidisciplinary approach is emphasized when treating the child with chronic pain. Future research in the paradigms for managing chronic pain in children needs to be conducted to shape treatment strategies and develop novel approaches to caring for this challenging group of patients.
References
- van Dijk M, de Boer JB, Koot HM, Tibboel D, Passchier J, Duivenvoorden HJ. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain 2000; 84: 367-77
- Sherry DD, Malleson PN. The idiopathic musculoskeletal pain syndromes in childhood. Rheum Dis Clin North Am 2002; 28: 669-85
- de Blecourt AC, Schiphorst Preuper HR, Van Der Schans CP, Groothoff JW, Reneman MF. Preliminary evaluation of a multidisciplinary pain management program for children and adolescents with chronic musculoskeletal pain. Disabil Rehabil 2008; 30: 13-20
- Goddard JM. Chronic pain in children and young people. Curr Opin Support Palliat Care 2011; 5: 158-6
- Kato J, Gokan D, Ueda K, Shimizu M, Suzuki T, Ogawa S. Successful pain management of primary and independent spread sites in a child with CRPS type I using regional nerve blocks. Pain Med 2011; 12: 17
- King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 2011; 152: 2729-3
- McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children's pain: an initial validation study. Pain 1996; 64: 435-43
- Varni JW, Rapoff MA, Waldron SA, Gragg RA, Bernstein BH, Lindsley CB. Chronic pain and emotional distress in children and adolescents. J Dev Behav Pediatr 1996; 17: 154-61
- McGrath PJ, Beyer J, Cleeland C, Eland J, McGrath PA, Portenoy R. American Academy of Pediatrics Report of the Subcommittee on Assessment and Methodologic Issues in the Management of Pain in Childhood Cancer. Pediatrics 1990; 86: 814-7
- Varni JW, Thompson KL, Hanson V. The Varni/Thompson Pediatric Pain Questionnaire. I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain 1987; 28: 27-38
- Jacobson CJ, Farrell JE, Kashikar-Zuck S, Seid M, Verkamp E, Dewitt EM. Disclosure and self-report of emotional, social, and physical health in children and adolescents with chronic pain--a qualitative study of PROMIS pediatric measures. J Pediatr Psychol 2013; 38: 82-93
- Jaworski TM, Bradley LA, Heck LW, Roca A, Alarcon GS. Development of an observation method for assessing pain behaviors in children with juvenile rheumatoid arthritis. Arthritis Rheum 1995; 38: 1142-51
- Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain 2004; 107: 213-9
- Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain 2006; 121: 77-84
- Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain 2011; 152: 1600-7
- Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of pediatric psychology 1991; 16: 39-58
- Palermo TM, Witherspoon D, Valenzuela D, Drotar DD. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain 2004; 109: 461-70
- Walker LS, Beck JE, Garber J, Lambert W. Children's Somatization Inventory: psychometric properties of the revised form (CSI-24). J Pediatr Psychol 2009; 34: 430-40
- Harter S, Pike R. The pictorial scale of perceived competence and social acceptance for young children. Child Dev 1984; 55: 1969-82
- Eccleston C, Morley S, Williams A, Yorke L, Mastroyannopoulou K. Systematic review of randomised controlled trials of psychological therapy for chronic pain in children and adolescents, with a subset meta-analysis of pain relief. Pain 2002; 99: 157-65
- Logan DE, Conroy C, Sieberg CB, Simons LE. Changes in willingness to self-manage pain among children and adolescents and their parents enrolled in an intensive interdisciplinary pediatric pain treatment program. Pain 2012; 153: 1863-70
- Yazdani S, Zeltzer L. Treatment of chronic pain in children and adolescents. Pain Manag 2013; 3: 303-14
- Vinson R, Yeh G, Davis RB, Logan D. Correlates of complementary and alternative medicine use in a pediatric tertiary pain center. Acad Pediatr 2014; 14: 491-6
- Cassileth BR, Vickers AJ. Massage therapy for symptom control: outcome study at a major cancer center. J Pain Symptom Manage 2004; 28: 244-9
- Palermo TM, Eccleston C, Lewandowski AS, Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain 2010; 148: 387-97
- van Tilburg MA, Chitkara DK, Palsson OS, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 2009; 124: e890-7
- Vlieger AM, Menko-Frankenhuis C, Wolfkamp SC, Tromp E, Benninga MA. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: a randomized controlled trial. Gastroenterology 2007; 133: 1430-6
- Zeltzer LK, Tsao JC, Stelling C, Powers M, Levy S, Waterhouse M. A phase I study on the feasibility and acceptability of an acupuncture/hypnosis intervention for chronic pediatric pain. J Pain Symptom Manage 2002; 24: 437-46
- Lin YC, Lee AC, Kemper KJ, Berde CB. Use of complementary and alternative medicine in pediatric pain management service: a survey. Pain Med 2005; 6: 452-8
- Tsao JC, Meldrum M, Kim SC, Jacob MC, Zeltzer LK. Treatment Preferences for CAM in children with chronic pain. Evidence-based complementary and alternative medicine : eCAM 2007; 4: 367-74
- Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain 1999; 81: 147-54
- Low AK, Ward K, Winnie AP. Pediatric complex regional pain syndrome. J Pediatr Orthop 2007; 27: 567-72
- Fitze G. [Complex regional pain syndrome in children]. Der Unfallchirurg 2011; 114: 411-6
- Harris EJ, Schimka KE, Carlson RM. Complex regional pain syndrome of the pediatric lower extremity: a retrospective review. J Am Podiatr Med Assoc 2012; 102: 99-104
- Guler-Uysal F, Basaran S, Geertzen JH, Goncu K. A 2 1/2-year-old girl with reflex sympathetic dystrophy syndrome (CRPS type I): case report. Clin Rehabil 2003; 17: 224-7
- Wilder RT, Berde CB, Wolohan M, Vieyra MA, Masek BJ, Micheli LJ. Reflex sympathetic dystrophy in children. Clinical characteristics and follow-up of seventy patients. J Bone Joint Surg Am 1992; 74: 910-9
- Martinez-Silvestrini JA, Micheo WF. Complex regional pain syndrome in pediatric sports: a case series of three young athletes. Bol Asoc Med P R 2006; 98: 31-7
- Gierthmuhlen J, Maier C, Baron R, et al. Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 2012; 153: 765-74
- Kurvers HA. Reflex sympathetic dystrophy: facts and hypotheses. Vasc Med) 1998; 3: 207-14
- Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain 2007; 131: 153-61
- Sarikaya A, Sarikaya I, Pekindil G, Firat MF, Pekindil Y. Technetium-99m sestamibi limb scintigraphy in post-traumatic reflex sympathetic dystrophy: preliminary results. Eur J Nucl Med 2001; 28: 1517-22
- Harden RN, Cole PA. New developments in rehabilitation of neuropathic pain syndromes. Neurol Clin 1998; 16: 937-50
- Karakaya I, Coskun A, Agaoglu B, Iseri P, Inanir M, Canatay H. Psychiatric approach in the treatment of reflex sympathetic dystrophy in an adolescent girl: a case report. Turk J Pediatr 2006; 48: 369-72
- Cho S, McCracken LM, Heiby EM, Moon DE, Lee JH. Pain acceptance-based coping in complex regional pain syndrome Type I: daily relations with pain intensity, activity, and mood. J Behav Med 2013; 36: 531-
- Kesler RW, Saulsbury FT, Miller LT, Rowlingson JC. Reflex sympathetic dystrophy in children: treatment with transcutaneous electric nerve stimulation. Pediatrics 1988; 82: 728-32
- Bialocerkowski AE, Daly A. Is physiotherapy effective for children with complex regional pain syndrome type 1? Clin J Pain 2012; 28: 81-91
- Richeimer SH, Bajwa ZH, Kahraman SS, Ransil BJ, Warfield CA. Utilization patterns of tricyclic antidepressants in a multidisciplinary pain clinic: a survey. Clin J Pain 1997; 13: 324-9
- Burgess CD, Montgomery S, Wadsworth J, Turner P. Cardiovascular effects of amitriptyline, mianserin, zimelidine and nomifensine in depressed patients. Postgrad Med J 1979; 55: 704-8
- Prensky A. Childhood Migraine Headache Syndromes. Current treatment options in neurology 2001; 3: 257-70
- Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005; 115: 254-63
- Smith AJ. The analgesic effects of selective serotonin reuptake inhibitors. J Psychopharmacol 1998; 12: 407-13
- Meighen KG. Duloxetine treatment of pediatric chronic pain and co-morbid major depressive disorder. J Child Adolesc Psychopharmacol 2007; 17: 121-7
- Farid IS, Heiner EJ. Intrathecal local anesthetic infusion as a treatment for complex regional pain syndrome in a child. Anesth Analg 2007; 104: 1078-80
- Arner S. Intravenous phentolamine test: diagnostic and prognostic use in reflex sympathetic dystrophy. Pain 1991; 46: 17-22
- Suresh S, Wheeler M, Patel A. Case series: IV regional anesthesia with ketorolac and lidocaine: is it effective for the management of complex regional pain syndrome 1 in children and adolescents? Anesth Analg 2003; 96: 694-5
- Dadure C, Motais F, Ricard C, Raux O, Troncin R, Capdevila X. Continuous peripheral nerve blocks at home for treatment of recurrent complex regional pain syndrome I in children. Anesthesiology 2005; 102: 387-91
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg 2007; 105: 1234-42
- Meier PM, Zurakowski D, Berde CB, Sethna NF. Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology 2009; 111: 372-80
- Maihofner C, Seifert F, Markovic K. Complex regional pain syndromes: new pathophysiological concepts and therapies. Eur J Neurol 2010; 17: 649-60
- Zernikow B, Dobe M, Hirschfeld G, Blankenburg M, Reuther M, Maier C. [Please don't hurt me!: a plea against invasive procedures in children and adolescents with complex regional pain syndrome (CRPS)]. Schmerz 2012; 26: 389-95
- Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I). Eur J Pain 2008; 12: 53-9
- Ashwal S, Tomasi L, Neumann M, Schneider S. Reflex sympathetic dystrophy syndrome in children. Pediatr Neurol 1988; 4: 38-42
- Bille BS. Migraine in school children. A study of the incidence and short-term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta Paediatr Suppl 1962; 136: 1-151
- Wang SJ, Juang KD, Fuh JL, Lu SR. Psychiatric comorbidity and suicide risk in adolescents with chronic daily headache. Neurology 2007; 68: 1468-73
- Bellini B, Arruda M, Cescut A, et al. Headache and comorbidity in children and adolescents. J Headache Pain 2013; 14: 79
- Mack KJ. An approach to children with chronic daily headache. Dev Med Child Neurol 2006; 48: 997-1000
- Meier PM, Alexander ME, Sethna NF, De Jong-De Vos Van Steenwijk CC, Zurakowski D, Berde CB. Complex regional pain syndromes in children and adolescents: regional and systemic signs and symptoms and hemodynamic response to tilt table testing. Clin J Pain 2006; 22: 399-406
- Hershey A, Kabbouche M, Powers S. Tension-type headache in the young. Curr Pain Headache Rep 2006; 10: 467-70
- Lewis DW. Headaches in children and adolescents. Curr Probl Pediatr Adolesc Health Care 2007; 37: 207-46
- Dooley JM, Gordon KE, Wood EP, Brna PM, MacSween J, Fraser A. Caffeine as an adjuvant to ibuprofen in treating childhood headaches. Pediatr Neurol 2007; 37: 42-6
- Greher M, Moriggl B, Curatolo M, Kirchmair L, Eichenberger U. Sonographic visualization and ultrasound-guided blockade of the greater occipital nerve: a comparison of two selective techniques confirmed by anatomical dissection. Brit J Anaesth 2010; 104: 637-42
- Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol 2004; 18: 613-31
- Brett T, Rowland M, Drumm B. An approach to functional abdominal pain in children and adolescents. Br J Gen Pract 2012; 62: 386-7
- Saps M, Hudgens S, Mody R, Lasch K, Harikrishnan V, Baum C. Seasonal patterns of abdominal pain consultations among adults and children. J Pediatr Gastroenterol Nutr 2013; 56: 290-6
- Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012; 153: 1798-806
- Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain 2010; 150: 568-72
- Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009; 137: 1261-9
- Pak T, Mickelson J, Yerkes E, Suresh S. Transverse abdominis plane block: a new approach to the management of secondary hyperalgesia following major abdominal surgery. Paediatr Anaesth 2009; 19: 54
- Kehlet H. Chronic pain after groin hernia repair. Br J Surg 2008; 95: 135-6
- Suresh S, Patel A, Porfyris S, Ryee MY. Ultrasound-guided serial ilioinguinal nerve blocks for management of chronic groin pain secondary to ilioinguinal neuralgia in adolescents. Paediatr Anaesth2008; 18: 775-
- Houlahan KE, Branowicki PA, Mack JW, Dinning C, McCabe M. Can end of life care for the pediatric patient suffering with escalating and intractable symptoms be improved? J Pediatr Oncol Nurs 2006; 23: 45-5
- Miser AW, Dothage JA, Wesley RA, Miser JS. The prevalence of pain in a pediatric and young adult cancer population. Pain 1987; 29: 73-8
- Miser AW, McCalla J, Dothage JA, Wesley M, Miser JS. Pain as a presenting symptom in children and young adults with newly diagnosed malignancy. Pain 1987; 29: 85-90
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med 2000; 342: 326-3
- Ljungman G, Kreuger A, Gordh T, Sorensen S. Pain in pediatric oncology: do the experiences of children and parents differ from those of nurses and physicians? Ups J Med Sci 2006; 111: 87-95
- Rork JF, Berde CB, Goldstein RD. Regional anesthesia approaches to pain management in pediatric palliative care: a review of current knowledge. J Pain Symptom Manage 2013; 46: 859-73
Leave a commentOrder by
Newest on top Oldest on top