Complex Regional Pain Syndrome
Revised March 17, 2015, by
Matthew Jaycox, MD
Assistant Professor of Anesthesiology
Timothy Lubenow, MD
Professor of Anesthesiology
Section of Pain Medicine
Rush Medical College
Rush University Medical Center
Chicago, IL
Contents
Introduction
Classification
Epidemiology
Pathophysiology
Clinical Diagnostic Criteria
Treatment
Prognosis
Summary
References
Introduction
The condition known as complex regional pain syndrome (CRPS) was first described during the American Civil War. Not coincidentally, as a result of advances in battlefield medicine during this time, soldiers were surviving from wounds that formerly would have claimed their lives. Yet, many carried with them a constant reminder of the war: severe, crippling, neurologic pain that long outlasted the healing of their wounds. This condition was termed “causalgia.”[1-2] Veterans were not the only ones affected. It was noted at the turn of the century that patients could develop similar sequelae following trivial injuries, which might evolve into osteoporosis and atrophy at the site of injury.[3-5] Rene Leriche was the first to implicate the sympathetic nervous system as a factor in the condition, and the term “reflex sympathetic dystrophy” (RSD) was introduced to reflect the proposed disruption in the sympathetic nervous system to the affected area.[6-7] Over time, many different names have been applied to the features that culminate in the syndrome, such as:
- Algodystrophy
- Algoneurodystrophy
- Causalgia
- Post-traumatic dystrophy
- Post-traumatic osteoporosis
- Post-traumatic pain syndrome
- Reflex sympathetic dystrophy
- Shoulder-hand syndrome
- Sudeck’s atrophy.
Classification
International Association for the Study of Pain (IASP) I and II: Prior to 1986, no formalized descriptive criteria existed for RSD. In 1986, the IASP proposed a formal classification of the condition.[8-9]However, the description lacked rigorous diagnostic criteria, and many neuropathic pain conditions were erroneously given the diagnosis of RSD, specifically those resistant to traditional treatments. In 1994, the IASP published diagnostic criteria for CRPS that focused on clinical diagnosis from patient history, symptom description, physical signs, and pain.[10] These diagnostic criteria were:
- The presence of an initiating noxious event or a cause of immobilization (Type I); or continuing pain or allodynia after a nerve injury, not necessarily limited to the distribution of the injured nerve (Type II)
- Continuing pain, allodynia, or hyperalgesia in which the pain is disproportionate to any known inciting event
- Evidence at some time of edema; changes in skin blood flow or abnormal sudomotor activity in the region of pain
- The diagnosis is excluded by the existence of other conditions that would otherwise account for the degree of pain and dysfunction.
This new taxonomy divided CRPS into Type I and Type II, distinguished by their inciting events. Type I (formerly RSD) follows a soft tissue injury, and CRPS II (causalgia) follows a well-defined nerve injury. The new term encompassed the many facets of the syndrome, including the complexity of the varied presentations; the distribution of symptoms; the presence of pain usually out of proportion to the inciting trauma; and the fact that it is a syndrome, which denotes the constellation of signs and symptoms. CRPS specifically addresses the varied contribution of the sympathetic nervous system.
Still, the revised IASP criteria suffered a lack of specificity, meaning that although they reliably identified many cases of CRPS, many non-CRPS cases were given the diagnosis incorrectly.[37] This lack of specificity stemmed from the fact that the diagnostic IASP criteria for CRPS could be met entirely based on patient reporting of symptoms, allowing the clinician great leeway in interpreting whether the condition was out of proportion to injury.[86]
Budapest Criteria: In direct response to these limitations, an international consensus panel was convened in Budapest in 2003, with the goal of recommending improvements to the IASP criteria. These modified criteria, henceforth known as the "Budapest Criteria," mandated that both historical and physical exam features in the following four key areas be present at the time of diagnosis.
- At least one sign at time of evaluation in two or more of the following categories
- Sensory: Reports of hyperesthesia and/or allodynia
- Vasomotor: Reports of temperature asymmetry and/or skin color changes and/or skin color asymmetry
- Sudomotor/edema: Reports of edema and/or sweating changes and/or sweating asymmetry
- Motor/trophic: Reports of decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin)
- Continuing pain, disproportionate to any inciting event
- At least one symptom in three of the four following categories
- Sensory: Evidence of hyperalgesia (to pinprick) and/or allodynia (to light touch and/or deep somatic pressure and/or joint movement)
- Vasomotor: Evidence of temperature asymmetry and/or skin color changes and/or asymmetry
- Sudomotor/edema: Evidence of edema and/or sweating changes and/or sweating asymmetry
- Motor/trophic: Dvidence of decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin)
- No other diagnosis that better explains the signs and symptoms.
Although the criteria set forth had been based on criteria that was empirically derived and previously published, they had never all been brought together in a single, unified diagnostic schema. Research since its publication suggests that the Budapest criteria lead to greater diagnostic consistency between clinicians and fewer diagnoses of CRPS. Research also suggests that these criteria improve on the existing IASP criteria.[86] Clinicians most favor the Budapest criteria today.
Epidemiology
The incidence and prevalence of CRPS varies. A population-based study of CRPS calculated the overall incidence of CRPS to be 26.2 per 100,000 person years, with the incidence of CRPS I to be 5.46 per 100,000 person years at risk and a prevalence of 20.57 per 100,000.[11] The incidence of CRPS II has been reported at 0.82 per 100,000 person years at risk and prevalence of 4.2 per 100,000 person years.[12]
Pathophysiology
Considerable disagreement exists regarding the mechanisms underlying CRPS. Likely more than one mechanism can describe or explain the pathophysiologic features. What is clear is that CRPS has a slight female predominance and a predilection for the extremities following trauma, fractures, and orthopedic surgeries.[13] One observation regarding pathophysiology has been the marked upregulation of alpha-1 adrenoreceptors, which appears in the injured extremity. These newly-expressed alpha-1 receptors spread along skin, muscle, and nerve tissue. These then augment depolarization in nerve and muscle tissue, resulting in an amplification effect of any stimuli. This accounts for the increase in pain when a patient has an increase in either endogenous or exogenous catecholamines, such as during times of stress.
Other theories have been put forth implicating peripheral mechanisms as well as central mechanisms for CRPS. In CRPS II, biochemical, physiological, and morphological changes occur in the injured, primary afferent, neurons. The changes likely become permanent if the axotomized afferent neurons do not regenerate to their target tissue. This causes many dorsal root ganglion cells (DRG) with unmyelinated afferent fibers to die.[14] The loss of DRG cells leads to degeneration of the centrally projecting afferent axons and to denervation of dorsal horn neurons. This induces secondary changes in the central representations (in the spinal cord, brain stem, thalamus and forebrain).[14]
In contrast, although CRPS I presents with symptoms similar to those seen in CRPS II, it usually does not present with a preceding nerve injury. Jänig et al. hypothesized that in CRPS I, central representations of the sensory, autonomic, and somatomotor systems account for the clinical presentation.[14-15] Later work, however, has unified the theories, arguing that CRPS, particularly type I, is a systemic disease of neuronal systems; the somatosensory system, the sympathetic nervous system, the somatomotor system, and peripheral (vascular, inflammatory) systems.[16]
Clinical Features
CRPS is a painful and debilitating disorder primarily affecting one or more extremities. The key features in CRPS are spontaneous pain, allodynia, hyperalgesia, edema, temperature change, abnormal vasomotor and sudomotor activity, trophic changes, and motor dysfunction (see Table 1).
Table 1. Pain Terms and Definitions
| Term | Definition |
| Allodynia | Pain caused by a stimulus that does not normally provoke pain |
| Causalgia | A syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion, often combined with vasomotor and sudomotor dysfunction and later trophic changes |
| Hyeralgesia | An increased response to a stimulus which is normally painful |
| Hyperesthesia | Increased sensitivity to stimulation, excluding the special senses |
| Hyperpathia | A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold |
| Motor dysfunction | Weakness, tremor, dystonia or decreased range of motion at the affected site or extremity |
| Neuropathic pain | Pain initiated or caused by a primary lesion or dysfunction in the nervous system |
| Sudomotor changes | Edema and/or sweating changes and/or sweating asymmetry at the affected site or extremity |
| Trophic changes | Hair, nail, and/or skin changes at the affected site or extremity |
| Vasomotor changes | Temperature asymmetry and/or skin color changes and/or skin color asymmetry at the affected site or extremity |
Although CRPS most frequently affects the limbs, it can occur anywhere in the body. A CRPS-like syndrome may be observed in patients with certain neoplasms (e.g., lung, breast, central nervous system, and ovarian cancers) and in patients after myocardial infarction or strokes.[17]
Spontaneous Pain
Patients suffering from CRPS may describe burning, throbbing, squeezing, aching, or shooting pain localized deep in the tissue.[17] This usually follows tissue injury to an extremity but is characteristically disproportionate in severity, duration, and extent of that expected from the clinical course of the initial injury.[18] Pain can be sympathetically mediated (relieved by sympathetic blockade), sympathetically independent (not relieved by sympathetic blockade), or mixed. CRPS varies in quality from a deep ache to a sharp stinging or burning sensation. Often patients report that environmental (cold, humidity) and emotional (anxiety, stress) factors worsen the pain. Cutaneous hypersensitivity presents as pain on contact with clothing or exposure to a cool breeze. The involved extremity is often guarded, even from the examining physician, and neglect of hygiene is common in the affected limb.[17]
Evoked Pain
Patients frequently experience pain from innocuous tactile stimuli (allodynia) and have an increased response to painful stimuli (hyperalgesia). All patients suffer from hyperalgesia, predominantly to mechanical stimuli or on joint movement. One third (higher incidence in chronic stages) suffer from severe allodynia (brush-evoked pain), a hallmark of central nociceptive sensitization.[19]
Sudomotor Changes and Edema
Although sometimes absent at presentation, marked edema can develop in the affected limb and be severe enough to lead to functional loss of the limb. Edema in the painful region gives a glossy and smooth appearance to the skin (see Figure 1). Notable limb edema has been reported in 80% of CRPS patients.[20] Sudomotor abnormalities range from hyperhidrosis to bone-dry skin and have been reported in 53% of patients.[20]
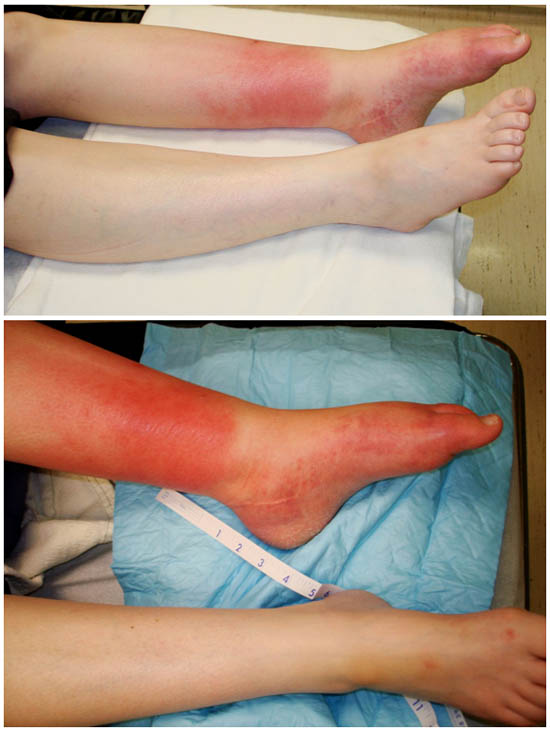
Figure 1. Equinovarus left foot, with sudomotor changes (glossy skin), vasomotor changes, motor dysfunction (“foot drop”), and allodynia in patient with CRPS for >5 years.
Temperature Change
The acute clinical presentation of CRPS is rubor, increased skin temperature, and edema.[21] [22] In the more chronic stages of CRPS, skin color becomes cyanotic and skin temperature decreases.[22] [23] Patients may describe the limb as mottled with dark, bluish, or pale white discoloration (see Figure 1).[18] These temperature changes are secondary to autonomic disturbance, which ranges from sympathetic hypofunction (hot, red, and dry) to sympathetic hyperfunction (cold, blue, pale or mottled, and sweaty). A difference in skin temperature (either higher or lower by 1°C) is found in 42% of patients with CRPS, and a difference of more than 2.2°C has a sensitivity of 76% and a specificity of 93% for CRPS.[24]
Motor Disturbances
Movement disturbance in the affected limb may present as tremulousness, weakness, decreased range of movement, muscle spasms, and dystonia. Dystonia in the upper extremity is typified by fingers in fixed flexion. Dystonia in the lower extremity often presents as an equinovarus position of the foot (see Figure 1).[17] Hand or foot dystonia develops in about 10% of patients. Range of movement may be compromised on the affected side, and contractures may develop in severe cases. Patients also may report decreased range of motion (80%) or motor weakness in the affected limb (75%). Tremors have been reported in approximately 50% of patients.[16] [25] Approximately 20% of patients display cyclonic action in the affected area.[20] [26] [27]
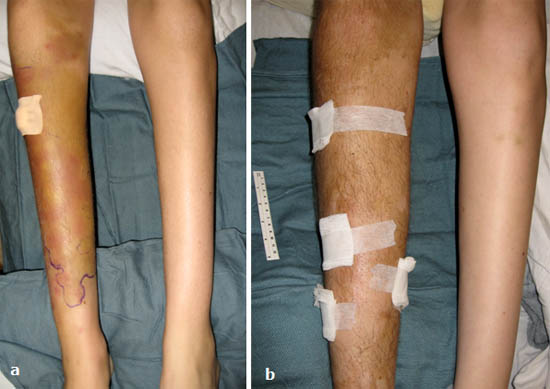 Figure 2. (a) Initial presentation with allodynia, vasomotor changes. (b) Six months later with allodynia (spontaneous and provoked), trophic changes (hair growth), sudomotor changes (edema), and motor dysfunction.
Figure 2. (a) Initial presentation with allodynia, vasomotor changes. (b) Six months later with allodynia (spontaneous and provoked), trophic changes (hair growth), sudomotor changes (edema), and motor dysfunction.
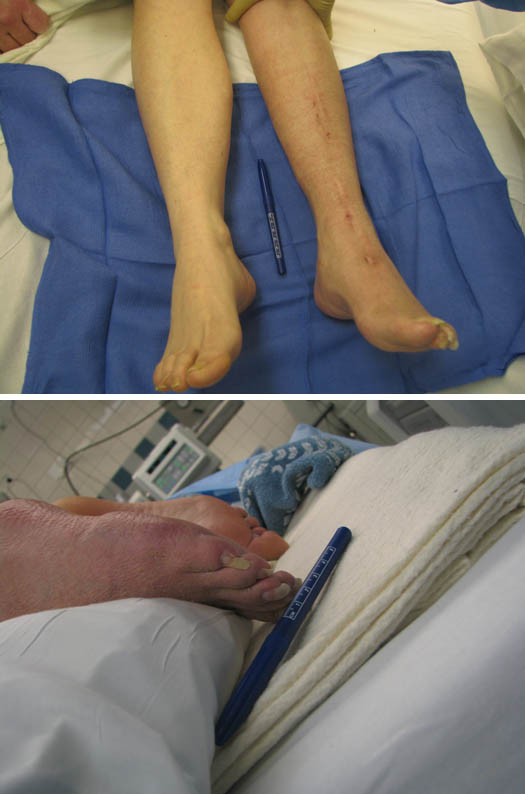 Figure 3. Initial presentation with allodynia, trophic changes, vasomotor changes, motor dysfunction (“foot drop”).
Figure 3. Initial presentation with allodynia, trophic changes, vasomotor changes, motor dysfunction (“foot drop”).
Trophic Changes
In latter stages of CRPS, fear for pain with movement can lead to disuse changes of the affected extremity, including osteoporosis visible by bone imaging.[18] Patients may present with trophic changes such as altered skin (hyperkeratosis), nail, or hair growth patterns (24%, 21%, and 18% of the patients, respectively) (see Figures 2 and 3).[20] Changes to skin are likely to be more common than nail or hair changes.
Clinical Stages
Classically, CRPS was subdivided into three distinct, sequential, progressive stages,[28] although recent work disputes this traditional staging and theorizes that patients are experiencing subtypes or subgroups. The classic stages are Stage I (the acute early, warm stage), marked by pain/sensory abnormalities (e.g., hyperalgesia, allodynia), vasomotor dysfunction, edema, and sudomotor disturbance. Stage II (dystrophic stage) is proposed to occur three to six months after onset and is characterized by intensified pain and sensory dysfunction, continued vasomotor disturbance, and development of motor and trophic changes. Stage III (atrophic stage) is characterized by a relatively cold extremity with decreased pain and sensory disturbance, continued vasomotor disturbance, and markedly increased motor and trophic changes.
This staging categorization carries much less significance today as CRPS is typically recognized earlier than in the past, many patients do not progress beyond stage I, or the timeframe in which they do advance is much more protracted than initially suggested. A multicenter cluster analysis was used to identify homogenous subgroups of patients with CRPS based on signs and symptoms and the duration of the disease.[29] The derived subgroups were statistically distinct and suggested three possible CRPS subtypes: (1) a relatively limited syndrome with vasomotor signs predominating; (2) a relatively limited syndrome with predominately neuropathic pain and sensory abnormalities; and (3) a florid CRPS syndrome similar to ‘‘classic RSD’’ descriptions[31]. The resulting CRPS subgroups did not differ significantly regarding pain duration, as might be expected in a sequential staging model. The IASP and Budapest diagnostic criteria acknowledge subgroups but do not make mention of the stages.
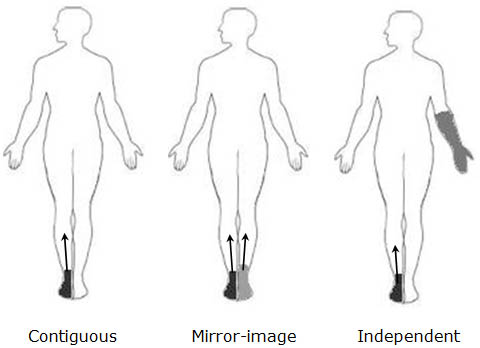 Figure 4. Three types of spread found in patients with CRPS, type I. Darkest area indicate initial site. Arrow indicate the direction of spread. Adapted from Maleki et al.[32]
Figure 4. Three types of spread found in patients with CRPS, type I. Darkest area indicate initial site. Arrow indicate the direction of spread. Adapted from Maleki et al.[32]
Pattern and Spread
CRPS does not affect a specific dermatome and the spread of CRPS is quite common.[30] Three patterns of spread have been characterized in the literature based on retrospective analysis (Figure 4). Contiguous spread (CS) is the most common and is characterized by a gradual and significant enlargement of the area affected initially. Independent spread (IS) is characterized by the appearance of CRPS in a location that was distant and non-contiguous with the initial site (e.g., CRPS appearing first in a foot, then in the hand). Mirror-image spread (MS) is the appearance of symptoms on the opposite side in an area that is closely matched in size and location to the site of initial presentation.[31] Schwartzman et al. reported CS in 31.1% of patients, MS in 11.5% of patients, ipsilateral extremity spread in 10.8% of patients, and contralateral extremity spread in 11.3% of patients.[30]
Predisposing Factors
CRPS is usually caused by trauma, including surgery, and is characterized by recognizable signs and symptoms. The inciting event (degree of trauma) may be trivial compared to the subsequent signs and symptoms, which are more severe than would normally be expected for the degree of trauma. CRPS affects women predominantly with a ratio approximating 2.0-3.5:1.[13][32][33] Its onset ranges from childhood through old age, but most cases are seen between 50 and 70 years of age. Dutch investigators generally believed that CRPS occurs mainly in Caucasian people, although this may reflect a population bias.[13][30][33][35] Schwartman et al. [30] reported that 77.6% of patients attributed injuries as the inciting event for CRPS. The most common causes of the injuries were motor vehicle accidents (23.6%), falls (14.6%), being struck by an object (3.4%), lifting heavy objects (3.2%), assault (2.2%), and medical procedures (1.6%). In 11.5% of the patients, surgery was listed as the initiating event. The majority of CRPS cases occurred after orthopedic surgical procedures. Estimates are 2.3%–4% after arthroscopic knee surgery, 2.1%–5% after carpal tunnel surgery, 13.6% after ankle surgery, 0.8%–13% after total knee arthroplasty, 7%–37% after wrist fractures, and 4.5%–40% after fasciectomy for Dupuytren contracture (see Table 2).[36] Other precipitating events include stroke (1.0%). In 7.8% of patients, no known initiating event was identified, although spontaneous CRPS is rare.[33]
Table 2. Estimated number of CRPS cases per orthopedic procedures
| Procedure | Number per year (in thousands) | Rate (%) | CRPS (number per year) |
|---|---|---|---|
| Arthroscopic knee surgery | 657 | 2.3-4.0 | 15.1-26.3 |
| Carpal tunnel surgery | 366 | 2.1-5.0 | 7.7-18.3 |
| Ankle fracture | 257 | 13.6 | 35.0 |
| Total knee arthroplasty | 247 | 0.8-13.0 | 2.0-32.1 |
| Wrist fracture | 194 | 7.0-37.0 | 13.6-71.8 |
| Fasciectomy for Dupuytren’s contracture | 20 | 4.5-40 | 0.9-8.0 |
| Total | 1,741 | 4.3-11.0 | 74.3-191.5 |
Clinical Diagnostic Criteria
As mentioned before, the diagnostic criteria set forth by IASP had a high sensitivity (0.98), but low specificity (0.36).[37] A low-specificity diagnostic tool leads to a high level of false positives and misdiagnoses. Many conditions mimic CRPS, and the lack of high diagnostic specificity may lead to inclusion, improper treatment, or delay in appropriate treatment.
The following should be used for differential diagnosis of CRPS.
- Fracture, sprain, strain
- Traumatic vasospasm
- Cellulitis
- Lymphedema
- Raynaud’s disease
- Thromboangiitis obliterans
- Erythromelalgia
- Deep vein thrombosis
The Budapest modifications to the IASP original criteria, allow a diagnosis of CRPS to be accurate in up to 88% of cases, and a diagnosis of non-CRPS neuropathic pain likely to be accurate in 97% of cases.[86] Additionally, the Budapest criteria retain the sensitivity of the IASP criteria (0.99), but with markedly higher specificity (0.68). Recent work has provided external validation of the Budapest Criteria as being superior to the IASP criteria[86] and suggest that it is likely the most rigorous diagnostic tool we have in identifying cases of CRPS.
A general definition of the syndrome CRPS describes an array of painful conditions that are characterized by a continuing (spontaneous or evoked) regional pain that is seemingly disproportionate in time or degree to the usual course of any known trauma or other lesion. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor, or trophic findings. The syndrome shows variable progression over time.
Debate exists regarding the best way to assess the various signs and symptoms necessary to make the diagnosis of CRPS. CRPS lacks a single objective test for its diagnosis, but a number of diagnostic tests may assist in determining the likelihood of the syndrome.
Diagnostic Examination
Sympathetic Blockade
Our approach to the diagnosis of this condition has evolved over the past three decades. In the 1970s, 1980s, and first half of the 1990s, pain relief to a selective sympathetic block was the primary diagnostic tool that physicians used to establish this diagnosis. Nonetheless, it is included here for historical purposes. Blockade of the sympathetic fibers had for a long time been used in the diagnosis and treatment of RSD. The IASP criteria published in 1994 removed the requirement that one need to demonstrate pain relief from a sympathetic block to confirm a diagnosis of RSD. However, the main utility of sympathetic blockade is to differentiate between sympathetically maintained pain and sympathetic-independent pain and to produce pain relief to facilitate physiotherapy.[39] This carries with it the secondary benefit that should a favorable response to sympathetic blockade be elicited, a useful treatment target is then identified. Delivering local anesthetic to sympathetic ganglion supplying the upper or lower extremity may provide valuable information, but the information should be interpreted with caution. Objective findings should be assessed after completion of medication delivery. Sudomotor, vasomotor, and sympathetic inhibition should be assessed. Local anesthetic spread to spinal nerve near the area of intended injection or systemic uptake can confound assessment of the blockade efficacy.[18]
Skin Temperature Measurement
In many patients with CRPS, a difference in temperature is noted between an affected extremity and the unaffected extremity. Infrared thermography has been used to evaluate temperature. A reported difference of more than 2.2°C has a sensitivity of 76% and a specificity of 93% for diagnosis of CRPS.[24] Breuhl and Lubenow et al reported a temperature difference of 0.8°C as the temperature asymmetry discriminating between CRPS and non-CRPS patients.[25]
Quantitative Autonomic Function Testing
The quantitative sudomotor axon reflex test (QSART) evaluates the difference in sweat production between an affected extremity and an unaffected extremity in response to a cholinergic challenge. The test assesses the integrity of both sides of the axon reflex arch. The use of the QSART test may help predict response to sympathetic blockade, as CRPS is clinically characterized by sensory, autonomic, and motor disturbances. Research needs to be conducted to further assess the utility of the test.
Vasomotor Testing
Acute CRPS may be manifested by an acute increase in vascular flow to the affected extremity secondary to neurogenic inflammation.[18] The decrease in sympathetic activity at the extremity may be measured by doppler flowmetry. As with QSART, the utility of vasomotor testing requires additional studies assess the utility in the diagnosis of CRPS.
Trophic Change Measurement
Chronic CRPS presents with changes in skin, nails, or bone. Evaluation of trophic changes to the bone by triple-phase bone scintigraphy has been used to substantiate the diagnosis of CRPS, although distinguishing between CRPS and acute trauma may difficult.[18]
Treatment
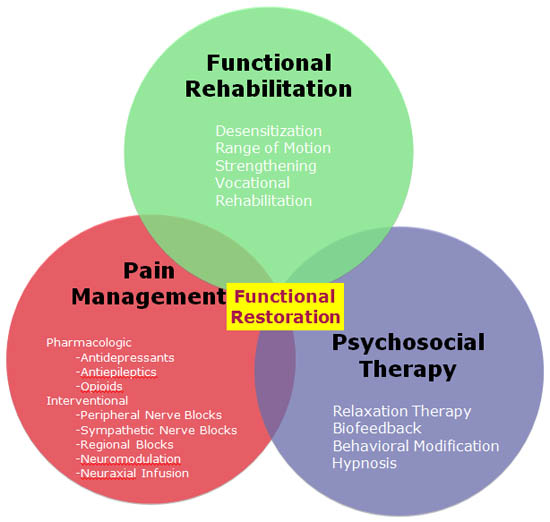
Figure 5. Therapeutic goals and strategies for the management of CRPS type I.
Pharmacological Therapy
The foundation of proper CRPS treatment lies in functional restoration, pain control, and psychotherapy. Therapeutic goals focus on optimizing the functional state of the extremity through physical therapy and psychotherapy, and on reducing stimulus-evoked pain and pain associated with extremity movement. The initiation of early functional restorative therapy is critical and correlates with improved outcomes. An algorithm has been proposed (see Figure 5) outlining treatment modalities, and therapy is directed at managing the signs and symptom of the disease.
Antidepressants
Both tricyclic and dual-inhibitor antidepressants have demonstrated efficacy in treating a variety of neuropathic pain conditions.[40][41] The effect of tricyclic antidepressants is multimodal. They antagonize calcium channels, sodium channels, and N-methyl-d-aspartate (NMDA) receptors on spinal cord dorsal horn neurons. Additionally, at this site, they inhibit reuptake of serotonin and norepinephrine, and this constellation of effects likely underlies their benefit.[40][42] Serotonin-norepinephrine reuptake inhibitors (SNRIs) inhibit the reuptake of both serotonin and norepinephrine and are often referred to as a dual inhibitors. The literature does not yet support their use in CRPS, though their success in treating post-herpetic neuralgia and diabetic peripheral neuropathy leads many to believe that these agents may reduce CRPS-associated pain. Although data is absent in the treatment of CRPS, the properties of the antidepressants may provide some symptom relief for the secondary consequences of the disease (e.g., an overweight, lethargic patient may benefit from an agent with more noradrenergic selectivity [desipramine] which may lead to appetite suppression). The sedating properties of amitriptyline also may be quite beneficial in patients with insomnia.[43][44]
Anticonvulsants (Antiepileptics)
Antiepileptics for the treatment of CRPS have shown mixed results. Gabapentin (GBP) and pregabalin (PGB) are the most prescribed anticonvulsant drugs in the treatment of CRPS. GBP modulates the voltage-gated α2δ−subunit of the calcium channels, but its true analgesic mechanism of action remains unknown. Mellick and Mellick[45] presented a case series of six patients in which GBP provided satisfactory pain relief, a reduction of hyperpathia, allodynia, hyperalgesia, reversal of skin and soft tissue manifestations, and improved sleep quality and sleep consolidation with far fewer nocturnal awakenings. In a prospective study, GBP was evaluated in 22 patients diagnosed with early stage CRPS.[46] The outcome measures were spontaneous visual analog scale (VAS), provoked VAS, range of motion, and edema. Statistically significant improvements in spontaneous and provoked VAS were found but not in the other measures. In contrast, van de Vusse[47] reported “mild effect on pain” with the use of GBP in patients with CRPS I.
Opioids
No long-term studies have been performed on oral opioids in the treatment of CRPS. Opioids should be considered in CRPS if pain limits the patient’s participation in physical restorative therapies designed to establish, maintain, or enhance function of the affected extremity. Although opioids may be less effective for chronic neuropathic pain conditions than for nociceptive pain, the data for opioid use do support improvements in the quality of life for patients with neuropathic pain.[44] [48] [49] Recently, Agarwal studied the effect of transdermal fentanyl on pain and function in three groups of patients suffering from neuropathic pain (e.g., small fiber or diabetic peripheral neuropathy), CRPS, and postamputation pain in a prospective, open-label trial.[50] Primary outcome measures included a change in pain intensity and daily activity and secondary outcomes included pain relief, cognition, physical function, and mood. All three groups reported significant decreases in pain at study conclusion. The CRPS group reported a reduction of 2.4 ± 0.40 (p < 0.001) from baseline on a 0-10 numerical rating scale. Moreover, the CRPS group experienced a 37.5% increase in daily activities compared to baseline.[50]
Calcium Regulating Medications (Bisphosphonates and Calcitonin)
Bisphosphonates and pyrophosphate analogues recently have been promoted as effective agents for the treatment of CRPS, but the mechanism of action is unknown. These compounds (alendronate, pamidronate, clodronate) may inhibit bone resorption, and their effectiveness have been confirmed in randomized controlled trials.[51-54] Calcitonin, a hormone secreted by the parafollicular cells of the thyroid gland, acts on bone and kidneys to inhibit osteoclastic bone resorption and thereby reduces serum calcium and phosphate.[55] Gobelet examined the efficacy of intranasal calcitonin in 63 patients with CRPS in a double-blind randomized study.[56] Significant reduction in both pain at rest and in motion, and increased mobility were reported. In a meta-analysis of pharmacologic treatments, Perez concluded that calcitonin could provide effective pain relief in CRPS patients.[57]
Free Radical Scavenger
Dimethylsulfoxide (DSMO) and N-acetylcysteine (NAC) also have been shown to be effective in treating CRPS.[58] The effectiveness and potency is variable, and their mechanism of action is unknown but may related to their antioxidant properties.
Ketamine
The NMDA receptor antagonist ketamine has been used in the treatment of CRPS, although its use is controversial. Currently, two dosing schema are used. The first is a low-dose “awake” regimen consisting of IV ketamine 25-100 mg per hour administered daily either as an inpatient or outpatient, repeated over several days (e.g., 5-10 days).[87-88] A recent placebo-controlled study by Schwartzman et al. (2009) administered ketamine to CRPS patients. The dose was 100 mg over four hours as an outpatient, repeated daily over 10 days. Outcome measures included thermal sensitivity, dynamic and static allodynia, finger motor function, and deep pressure pain thresholds. Patients were followed up at 12 weeks, and the ketamine group demonstrated statistically significant improvement in all primary outcome measures versus placebo.[88] Patients required coadministration of clonidine and midazolam to attenuate unwanted side effects from ketamine (e.g., hallucinations). A second dosing regimen involves periodically administering high-dose ketamine, 200-300 mg per hour in a monitored anesthetic setting. Practitioners in Germany have medically induced comas in CRPS patients to administer extremely high doses of ketamine (3–5 mg/kg per hour) over five days, subsequently managing the emergence delirium on an outpatient basis.[89] Such treatment is rarely reimbursed or approved by insurance companies in the United States.
Interventional Procedures
Sympathetic Nerve Blockade
Sympathetic blockade utilizing local anesthetics is performed for treatment of CRPS, not, as commonly believed, for diagnosis. Although poorly understood, the role of sympathetic nervous system dysfunction was previously presumed to be an essential component of the syndrome,[59] but there is growing debate regarding the degree to which the sympathetic nervous system contributes to the clinical syndrome.[60] A subset of CRPS patients may display sympathetically mediated pain and are more likely to receive pain relief from sympathetic blockade.
In a double-blind crossover study, Price investigated the effectiveness of local anesthetics in CRPS patients.[61] An immediate effect on pain and mechanical allodynia was found, but the response was similar in the control group (saline). In a Cochrane Review, Cepeda revealed the scarcity of published data to support the use of local anesthetic sympathetic blockade as the gold standard for CRPS treatment.[60] More recently Yucel evaluated the effectiveness of stellate ganglion blockade in CRPS.[62] The sympathetic blockade significantly improved VAS values and range of motion (ROM). Nerve blocks are recommended primarily to reduce pain and facilitate physiotherapy and functional rehabilitation.[4] Those who obtain pain relief and improved ROM should continue with an extended series of repeat blocks.
Epidural Infusion
Continuous epidural infusion, often with local anesthetic and opioid (e.g., bupivacaine and fentanyl), is an effective analgesic option in the treatment of CRPS.[63] The epidural catheter is placed sterilely under fluoroscopic guidance and aims to position the catheter tip on the affected side at the appropriate spinal segmental level. The catheter is tunneled under the skin for a distance of 3–5 inches and left in place for 5 days to 12 weeks.[63] [64] During infusion, the patient undergoes physiotherapy directed at restoration of function. Rauck et al. previously described in a randomized controlled fashion the efficacy of continuous epidural clonidine infusions for the treatment of refractory RSDD (CRPS) patients.[65]
Neuromodulation
Studies show that conventional pain medications, physical therapy, and sympathetic blockade all have less-than-favorable results for CRPS treatment.[65-67] Only one in five CRPS patients is capable of returning to a normal level of functioning.[65] Spinal cord stimulation (SCS) is an intervention modality that may be used in patients with refractory pain. The proposed mechanism of SCS began with the “gate theory” advanced by Melzack and Wall in 1965.[68] Specifically, the “gate” represents the termination of painful peripheral stimuli carried by C fibers (e.g., burning sensation) and thinly myelinated A-δ fibers (e.g., sharp, intense, tingling sensation) in the dorsal horn of the spinal cord. Large, myelinated A-β fibers (e.g., light touch, pressure, vibration, hair movement) also terminate in the dorsal horn. Melzack and Wall hypothesized that sensory input could be manipulated to close the “gate” to the transmission of painful stimuli. The mechanisms by which dorsal column stimulation modulate pain perception have yet to be elucidated; however, current understanding attributes pain reduction to the activation of large diameter afferent fibers (e.g., A-β fibers) by electrical stimulation.[69]
Symptoms of CRPS have been ranked the second most frequent indicator for SCS therapy in the United States (post-laminectomy pain syndrome being the first indication). Pain relief as high as 70% has been reported with neurostimulation (e.g., SCS or peripheral nerve stimulation) when patients are properly selected.[70-72] Spinal cord stimulation should be considered in the treatment algorithm when conservative therapies fail.
The literature supports the use of SCS in CRPS. For example, Kemler[65] studied the effectiveness of spinal cord stimulation and physical therapy versus physical therapy alone in CRPS affected patients. At six months, the SCS + physiotherapy group reported a significantly greater reduction in pain compared to the physiotherapy alone group. At 24 months, spinal cord stimulation resulted in improvement of long-term pain and health-related quality of life.[73] At five years, despite the diminishing effectiveness of SCS over time, 95% of patients with an implant would repeat the treatment for the same result.[74] Harke evaluated the long-term effect of SCS on functional improvement.[75] When SCS was combined with concurrent physiotherapy, a reduction in deep pain and allodynia along with improvement in functional status and quality of life were found.
Intrathecal Drug Delivery
Data citing the benefits of intrathecal drug delivery systems (IDDS) are limited, although case reports and series indicate benefit in CRPS patients.[76] An implantable pump is a viable consideration for patients who do not respond to SCS or have multiple sites of pain.[59] Intrathecal medications have long been established as effective agents for treating refractory cancer pain since 1979.[77] In a randomized control trial of 200 patients with advanced cancer and refractory pain, Smith demonstrated the effectiveness of intrathecal opioid in a group of patients receiving both IDDS and medical management compared to medical management alone.[78] The same has not been borne out in the treatment of CRPS. Alternatively ziconitide (PRIALT®, Elan Pharmaceuticals Inc., San Diego, CA), a nonopioid analgesic, has shown some promise in the treatment of severe chronic nonmalignant pain, including CRPS.[79] [80]
Prognosis
CRPS can be marked by significant pain and chronicity. Controversy in the literature exists, with reports of spontaneous resolution of CRPS in some patients,12 while many report continued symptoms[32] [81] A recent report indicates that an average of 5.8 years after the initiating injury, CRPS patients continue to have significantly higher symptom and sign prevalence rates when compared to reference patients with the same precipitating injury.[82] Most of the signs and symptoms became well established after one year, and might progress moderately with time.[30]
Prognostic factors for a good or poor outcome of CRPS are not known, although coldness of the affected limb has been associated with longer disease duration[83] and worse functional outcome.[26] [81] As with spontaneous resolution of symptoms, recurrence of symptoms may occur. It is a common belief that further trauma (e.g., surgery) to a previously affected extremity can reactivate CRPS, although this is not supported by literature data. The incidence of CRPS recurrence is estimated to be 10% or 1.8% per year at risk.[84] A five-year follow-up study in patients with CRPS involving the upper extremity indicated that 26% of patients had to change their jobs and nearly 30% of patients had to stop work for more than a year. However, 72% continued to work full time.[85]
Summary
CRPS is a painful and debilitating disorder primarily affecting one or more extremities. A specific etiology has not been identified, and the poor understanding of the underlying pathophysiological abnormalities contribute to the difficulties in diagnosis and treatment. No single diagnostic test or single or combination of therapies are universally effective for CRPS. Currently, effective treatment of chronic neuropathic pain continues to be a clinical challenge because of the variability in presentation. Treatment of CRPS focuses on an early, aggressive, multimodal approach that targets pain reduction and functional restoration. Presently, many of the medications used in the treatment of CRPS are approved for the treatment of other pain conditions. Continued research may reveal additional mechanisms of the disease leading to preventive measures and additional targets for drug activity.
References
- Mitchell S. Injuries of the nerves and their consequences. Philadelphia: JB Lippincott & Co.; 1872.
- Mitchell S. Gunshot wounds and other injuries of nerves. Philadelphia: JB Lippincot & Co.; 1864.
- Stanton-Hicks M. Complex Regional Pain Syndrome: A New Name for Reflex Sympathetic Dystrophy and Causalgia. Current Pain and Headache Reports 1997;1:34-40.
- Sudeck P. Nber die akute (reflectorishce) Knochenatrophie nach Zntzundungen und Verletzungern an den Extremitaten und Ihre Klinischen Erscheinungen. Fortschvr Geb Roentgenstr 1901;5:277-293.
- Gurd FB. Post-Traumatic Acute Bone Atrophy (Sudeck's). Ann Surg 1934;99:449-469.
- Leriche R. La Chirurgie del la Douleur. Paris: Massont C and Cie; 1939.
- Evans J. Reflex sympathetic dystrophy. Surg Clin North Am 1946;26:780-790.
- Merskey H. Classification of chronic pain: description of chronic pain syndromes and defnition of pain terms. Pain 1986;3:S215-S221.
- Harden RN, Bruehl S, Galer BS, et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain 1999;83:211-219.
- Merskey H, Bogduk, N. Classification of chronic pain. Seattle: IASP Press; 1994.
- de Mos M, de Bruijn AG, Huygen FJ, et al. The incidence of complex regional pain syndrome: a population-based study. Pain 2007;129:12-20.
- Sandroni P, Benrud-Larson LM, McClelland RL, et al. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103:199-207.
- Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain 1999;80:539-544.
- Janig W, Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clin Auton Res 2002;12:150-164.
- Blumberg H, Janig W. Changes of reflexes in vasoconstrictor neurons supplying the cat hindlimb following chronic nerve lesions: a model for studying mechanisms of reflex sympathetic dystrophy? J Auton Nerv Syst 1983;7:399-411.
- Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol 2003;2:687-697.
- Raja SN, Grabow TS. Complex regional pain syndrome I (reflex sympathetic dystrophy). Anesthesiology 2002;96:1254-1260
- Grabow TS, Guarino, A. H., Raja, S. N. Complex regional pain syndrome: Diagnosis and treatment. In: Benzon HT, Raja, S. N., Molloy, R. E., Liu, S. S., Fishman, S. M., ed. Essentials of pain medicine and regional anesthesia. 2nd ed. Philadelphia: Elsevier Churchill Livingston; 2005:380-385.
- Birklein F, Handwerker HO. Complex regional pain syndrome: how to resolve the complexity? Pain 2001;94:1-6.
- Harden RN, Bruehl SP. Diagnosis of complex regional pain syndrome: signs, symptoms, and new empirically derived diagnostic criteria. Clin J Pain 2006;22:415-419.
- Kurvers HA, Jacobs MJ, Beuk RJ, et al. Reflex sympathetic dystrophy: evolution of microcirculatory disturbances in time. Pain 1995;60:333-340.
- Birklein F, Riedl B, Neundorfer B, et al. Sympathetic vasoconstrictor reflex pattern in patients with complex regional pain syndrome. Pain 1998;75:93-100.
- Bej MD, Schwartzman RJ. Abnormalities of cutaneous blood flow regulation in patients with reflex sympathetic dystrophy as measured by laser Doppler fluxmetry. Arch Neurol 1991;48:912-915.
- Wasner G, Schattschneider J, Baron R. Skin temperature side differences--a diagnostic tool for CRPS? Pain 2002;98:19-26.
- Bruehl S, Lubenow TR, Nath H, Ivankovich AD: Validation of Thermography in the Diagnosis of Reflex Sympathetic Dystrophy and Non-RSD Chronic Pain Patients. Clinical Journal of Pain 12:316-325, 1996
- Deuschl G, Blumberg H, Lucking CH. Tremor in reflex sympathetic dystrophy. Arch Neurol 1991;48:1247-1252.
- van der Laan L, Veldman PH, Goris RJ. Severe complications of reflex sympathetic dystrophy: infection, ulcers, chronic edema, dystonia, and myoclonus. Arch Phys Med Rehabil 1998;79:424-429.
- Schwartzman RJ, Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology 1990;40:57-61.
- Bonica J. Causalgia and other reflex sympathetic dystrophies. In: Bonica J, ed. Management of pain. 2nd ed. Philadelphia: Lea & Febiger; 1990:220-243.
- Bruehl S, Harden RN, Galer BS, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain 2002;95:119-124.
- Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain 2009;25:273-280.
- Maleki J, LeBel AA, Bennett GJ, et al. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy). Pain 2000;88:259-266.
- Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 1993;342:1012-1016.
- de Mos M, Sturkenboom, M, Huygen, F. Current understanding on complex regional pain syndrome. Pain Practice 2009;9:86-99.
- Burns AW, Parker DA, Coolican MR, et al. Complex regional pain syndrome complicating total knee arthroplasty. J Orthop Surg (Hong Kong). 2006;14:280-283.
- Harden RN, Bruehl S, Stanos S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain 2003;106:393-400.
- Gottschalk A, Raja SN. Severing the link between acute and chronic pain: the anesthesiologist's role in preventive medicine. Anesthesiology 2004;101:1063-1065.
- Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain 1999;81(1-2):147-154.
- Harden RN, Bruehl, S. Diagnostic criteria: the statistical derivation of the four criterion factors. In: Wilson P, Stanton-Hicks, M, Harden, R.N., ed. CRPS: Current Diagnosis and Therapy. Seattle: IASP Press; 2005:45-58.
- Williams K, Hurley, R., Lin, E.E. Wu, C. L. Neuropathic pain syndromes. In: Benzon HT, Rathmell, JP, Wu, CL, Turk, DC, ed. Raj's practical management of pain'. 4th ed. Philadelpia: Mosby Elsevier; 2008.
- Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-409.
- Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2002;59:1015-1021.
- Colombo B, Annovazzi PO, Comi G. Medications for neuropathic pain: current trends. Neurol Sci 2006;27 Suppl 2:S183-189.
- Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-1256.
- Mackey S, Feinberg S. Pharmacologic therapies for complex regional pain syndrome. Curr Pain Headache Rep 2007;11:38-43.
- Mellick GA, Mellick LB. Reflex sympathetic dystrophy treated with gabapentin. Arch Phys Med Rehabil 1997;78:98-105.
- Tan AK, Duman I, Taskaynatan MA, et al. The effect of gabapentin in earlier stage of reflex sympathetic dystrophy. Clin Rheumatol 2007;26:561-565.
- van de Vusse AC, Stomp-van den Berg SG, Kessels AH, et al. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type 1 [ISRCTN84121379]. BMC Neurol 29 2004;4:13.
- Dellemijn P. Are opioids effective in relieving neuropathic pain? Pain 1999;80:453-462.
- Watson CP, Moulin D, Watt-Watson J, et al. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain 2003;105:71-78.
- Agarwal S, Polydefkis M, Block B, et al. Transdermal fentanyl reduces pain and improves functional activity in neuropathic pain states. Pain Med 2007;8:554-562.
- Manicourt DH, Brasseur JP, Boutsen Y, et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 2004;50:3690-3697.
- Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 2004;5:276-280.
- Varenna M, Zucchi F, Ghiringhelli D, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 2000;27:1477-1483.
- Adami S, Fossaluzza V, Gatti D, et al. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis 1997;56:201-204.
- Sharma A, Williams K, Raja SN. Advances in treatment of complex regional pain syndrome: recent insights on a perplexing disease. Curr Opin Anaesthesiol 2006;19:566-572.
- Gobelet C, Waldburger M, Meier JL. The effect of adding calcitonin to physical treatment on reflex sympathetic dystrophy. Pain 1992;48:171-175.
- Perez RS, Kwakkel G, Zuurmond WW, et al. Treatment of reflex sympathetic dystrophy (CRPS type 1): a research synthesis of 21 randomized clinical trials. J Pain Symptom Manage 2001;21:511-526.
- Perez RS, Zuurmond WW, Bezemer PD, et al. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain 2003;102:297-307.
- Nelson DV, Stacey BR. Interventional therapies in the management of complex regional pain syndrome. Clin J Pain 2006;22:438-442.
- Cepeda MS, Carr DB, Lau J. Local anesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database Syst Rev 2005:CD004598.
- Price DD, Long S, Wilsey B, et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain 1998;14:216-226.
- Yucel I, Demiraran Y, Ozturan K, et al. Complex regional pain syndrome type I: efficacy of stellate ganglion blockade. J Orthop Traumatol 2009;10:179-183.
- Moufawad S, Malak, O., Mekhail, N. Epidural infusion of opiates and local anesthetics for complex regional pain syndrome. Pain Pract 2002;2:81-86.
- Rauck, R. L., Eisenach, J. C., Jackson, K., Young, L. D., & Southern, J. (1993). Epidural clonidine treatment for refractory reflex sympathetic dystrophy.Anesthesiology, 79(6), 1163-1169.
- Schwartzman RJ, Patel M, Grothusen JR, et al. Efficacy of 5-day continuous lidocaine infusion for the treatment of refractory complex regional pain syndrome. Pain Med 2009;10:401-412.
- Kemler MA, Barendse GA, van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 2000;343:618-624.
- Grabow TS, Tella PK, Raja SN. Spinal cord stimulation for complex regional pain syndrome: an evidence-based medicine review of the literature. Clin J Pain 2003;19:371-383.
- Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage 2006;31:S13-19.
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971-979.
- Williams B, Christo, P. Pharmacological and interventional treatment of neuropathic pain. In: Dobretsov M, Zhang, J.M., ed. Mechanisms of pain in peripheral neuropathy. Kerala: Research Signpost; 2009.
- Stanton-Hicks M, Baron R, Boas R, et al. Complex Regional Pain Syndromes: guidelines for therapy. Clin J Pain 1998;14:155-166
- Barolat G, Sharan AD. Future trends in spinal cord stimulation. Neurol Res 2000;22:279-284.
- Fogel GR, Esses SI, Calvillo O. Management of chronic limb pain with spinal cord stimulation. Pain Pract 2003;3:144-151.
- Kemler MA, De Vet HC, Barendse GA, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years' follow-up of the randomized controlled trial. Ann Neurol 2004;55:13-18.
- Kemler MA, de Vet HC, Barendse GA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome Type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg 2008;108:292-298.
- Harke H, Gretenkort P, Ladleif HU, et al. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain 2005;9:363-373.
- Zuniga RE, Perera, S., Abram, S.E. Intrathecal baclofen: a useful agent in the treatment of wel-established complex regional pain syndrome. Reg Anesth Pain Med 2002;27:90-93.
- Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology 1979;50:149-151.
- Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol 2002;20:4040-4049.
- Wallace MS, Charapata, S.G., Fisher, R., Byas-Smith, M., Staats, P.S., Mayo, M., McGuire, D., Ellis, D. Intrathecal ziconitide in the treatment of chronic nonmalignant pain: a randomized, double-blind, placebo-controlled clinical trial. Neuromod. 2006;9:75-86.
- Kapural L, Lokey K, Leong MS, et al. Intrathecal ziconotide for complex regional pain syndrome: seven case reports. Pain Pract 2009;9:296-303.
- Galer BS, Henderson J, Perander J, et al. Course of symptoms and quality of life measurement in Complex Regional Pain Syndrome: a pilot survey. J Pain Symptom Manage 2000;20:286-292.
- de Mos M, Huygen FJ, van der Hoeven-Borgman M, et al. Outcome of the complex regional pain syndrome. Clin J Pain 2009;25:590-597.
- Wasner G, Schattschneider J, Heckmann K, et al. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain 2001;124(Pt 3):587-599.
- Veldman PH, Goris RJ. Multiple reflex sympathetic dystrophy. Which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain Mar 1996;64:463-466.
- Geertzen JH, Dijkstra PU, Groothoff JW, et al. Reflex sympathetic dystrophy of the upper extremity--a 5.5-year follow-up. Part II. Social life events, general health and changes in occupation. Acta Orthop Scand Suppl 1998;279:19-23.
- Harden RN, Bruehl S, Stanton-Hicks M, et al. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. May-Jun 2007;8(4):326-331.
- Goldberg ME J, Domsky R, Scaringe D, et al. Multi-day low dose ketamine infusion for the treatment of complex regional pain syndrome. Pain Physician. Apr 2005 8(2):175-179.
- Schwartzman RJ, Alexander GM, Grothusen JR, et al. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo conbtrolled study. Pain. Dec 2009 15;147(1-3):107-15.
- Kiefer RT, Rohr P, Ploppa A, et al. Complete recovery from complex regional pain syndrome, CRPS type I, following anesthetic ketamine and midazolam. Pain Practice. June 2007;7(2):147-50.
Leave a commentOrder by
Newest on top Oldest on top