Discogenic Pain and Thermal Annular Procedures
Authors
Fnu Kailash, MD
Fellow Physician
Salim M Hayek, MD, PhD
Associate Professor
Chief, Division of Pain Medicine
Joshua Goldner, MD
Assistant Professor
Department of Anesthesiology
Case Western Reserve University
University Hospitals Case Medical Center
Cleveland, OH
Copyright ©2010 Covidien. All rights reserved. Reprinted with the permission of the Energy Based Devices Division of Covidien
Introduction
The lifetime prevalence of low back pain (LBP) has been reported as between 54% and 80%.[1] LBP is one of the most common reasons for visits to internists and the second most common reason for disability in the United States.[2-3] The prevalence of chronic low back pain ranges from 15-45%, with a median point prevalence of 30%.[1] It is the most significant source of spine-related pain and is associated with significant economic, societal and health implications. The impact of low back pain on the US economy can be gauged from the fact that spine-related health care expenditure reached around $86 billion for the year 2005, increasing 65% since 1997.[4] The increase in health care utilization among those with LBP is multifactorial, including an increased prevalence of acute and chronic LBP, increased prevalence of those with chronic LBP who seek care, increased per-user health care cost, and innovation in interventional techniques.[4-6]
The true burden of low back pain – both personally and economically – comes not from those who suffer from acute low back pain, but those who deal with its chronic variant. It has long been thought that 80-90% of acute episodes of low back pain will resolve in about six weeks with 5-10% of cases progressing to persistent pain.[7] More recent studies show evidence to the contrary. That is, a significant minority (as many as two third) of patients will experience relapses and recurrent episodes of long lasting pain. In a 2008 study, Stanton et al. reported recurrence rates of low back pain as high as 24% to 33%.[8]
Diagnosis of Spine Pain
In interventional pain medicine, clinicians are often faced with a dilemma in diagnosing the cause of spinal pain. Based on history, physical examination, radiologic imaging and neurophysiologic studies, only 15% of the cases of low back pain are identified in the absence of neurologic deficits and clear disc herniation. Controlled diagnostics blocks can identify the cause of pain in 85% of cases with non-radicular or discogenic pain.[6] The premise of diagnostic blocks has been elaborated by Bogduk.[9]
- The structure to be considered as a cause of pain is innervated
- The structure should be able to reproduce the pain similar to that seen in clinical situations
- The structure can be subjected to disease or pathology which is painful
- The structure should have been shown to be a source of pain in patients, using diagnostic techniques of known reliability and validity
There are three structures which are considered to be most important causes of chronic LBP with best available scientific evidence and proven diagnostic techniques.[6] They are the intervertebral discs, facet joints and the sacroiliac joint. By employing controlled diagnostic injections, the relative contribution of these structures as a source of chronic LBP has been estimated at 39%,[10] 15%,[11] and 19%[12] respectively.
Discogenic Pain
Pathophysiology
Discogenic pain constitutes a major sub-group (≈40%) of chronic low back pain especially in the younger patient population.[10] Based on anatomical and histological studies, it has been established that the intervertebral disc is innervated by nociceptive nerve fibers in the outer third of the annulus fibrosus.[9] The pathophysiology of discogenic pain is not well understood. However, annular tears or disruptions, in-growth of the nociceptive nerve endings into the painful discs and multiple inflammatory mediators with sensitization of the nociceptors are likely contributing factors.[13-15]
Diagnosis
History and Physical Examination
Diagnostic clinical tests such as history, physical examination and radiologic imaging have low sensitivity and specificity in determining whether an intervertebral disc is in fact a pain generator.[16] Clinical manifestations of discogenic pain, though non-specific, are characterized by sitting intolerance, and worsening of pain under various spinal loading combinations especially in sitting and standing postures with forward flexion as corroborated by in-vivo intradiscal pressure measurements.[17] It has also been noted that maximum annular straining occurs in postero-lateral segment under complex spinal loading conditions.[18]
Imaging Studies
Amongst the various imaging modalities, MRI is the best non-invasive test to detect morphologic features of disc degeneration such as loss of nuclear signal, high intensity zones, and vertebral end plate changes. Despite its superior resolution and non-invasive nature, there is only a tenuous relationship between anatomic abnormality and pain generation. Some proponents of “high intensity zones” in the posterior annulus on T-2 weighted images have established its correlation with painful, severely disrupted disk on discography.[9][19-21] On the other hand, skeptics have frowned upon its diagnostic value, and its validity has been questioned by an undefined prevalence in the asymptomatic population.[22] In a systemic review of diagnostic tests to identify the disc as a source of back pain, Hancock et al.[16] concluded that centralization of radicular symptoms was the only clinical feature which increases the likelihood of a disc as a source of pain, and absence of disc degeneration on MRI was the only test to state otherwise.
Provocative Studies
Crock described internal disc disruption (IDD), a major source of discogenic pain, which signifies a alteration in the internal architecture of the disc with normal external morphology, with no evidence of disc prolapse or disc herniation.[23] Currently, provocation discography (disc stimulation) - which involves injection of non-ionic contrast medium into the disc with or without pressure manometry and post-discography computed tomography scan - is the only diagnostic test able to simultaneously detect a painful disc and give further information about the architecture of internal disc disruption.
Provocation Discography (Disc Stimulation)
Discography is an invasive procedure that is used to characterize the pathoanatomy of the intervertebral disc and determine if the intervertebral disc is the source of chronic LBP. The premise is: "If a particular disc is painful, then stressing it should reproduce the patient's usual pain. If the disc is not the source of a patient's pain, then stressing it either should not be painful or should produce pain that is not the patient's familiar or accustomed pain" (International Spine Intervention Society [ISIS] Guidelines).[24]
According to ISIS guidelines, the diagnostic criteria for positive discogram are:
- Pain more than 7/10
- Concordant pain with intradiscal pressure < 50 psi above opening pressure
- Grade III annular tear on modified Dallas classification
- At least one, preferably two painless control discs
This procedure is plagued by controversy in the literature with regards to its subjective nature of reporting pain and higher false positive rates in those with concurrent psychosomatic and other chronic pain disorders.[25-26] However, modern techniques incorporating accepted standards in the diagnostic paradigm can enhance its validity for diagnosing symptomatic discs with lower false positive rates.[27-28]
Given the contradictory studies with regards to its diagnostic validity and utility, and its invasive nature, discography would not be considered as an initial step in the treatment algorithm of the back pain.[29] Once facet joint pain, and if applicable sacroiliac joint pain, are ruled out as a cause of pain, and the patient fails to respond to at least one fluoroscopically guided epidural injections, then discography can be pursued in order to determine disc as a source of pain followed by initiation or change in patients treatment regimen including surgery or minimally invasive intradiscal therapies.
Treatment
Intradiscal Therapy
There are several techniques utilized for the treatment of discogenic back pain with or without disc herniation. Due to variable outcomes and their antecedent complications, traditional surgical techniques such as lumbar fusion and artificial disc replacement are being replaced by less invasive percutaneous intradiscal therapies. Dating back to the 1960’s, Lyman Smith was the first to perform percutaneous injection of chymopapain (a proteolytic enzyme) for unrelenting sciatica, a technique he called chymonucleolysis (CNL).[30] Although CNL fell out of the favor due to fatal allergic reactions, clinicians’ desire for minimally invasive therapies led to the birth of several percutaneous intradiscal therapies in the last 50 years.
Once the diagnosis of discogenic pain is made, the next step is the institution of effective therapy according to disc pathology. The extent of annular disruption is an important concept in the understanding of the IDD and discogenic pain. Sachs et al. developed the Dallas discogram scale that was a gold standard test for CT classification of annular tears.[31] This classification has been further modified by April and Bogduk in 1992.20 Annular disruption is graded on 5-point scale on modified Dallas discogram classification as follows:
- Grade 0 = contrast medium contained entirely within regular nucleus pulposus
- Grade I = extension of contrast medium to inner third of the annulus fibrosus
- Grade II = extension of contrast medium to middle third of the annulus fibrosus
- Grade III = extension of contrast medium to outer third of the annulus fibrosus
- Grade IV = Grade III plus extension of contrast medium circumferentially around the annulus fibrosus by at least 30°
- Grade V = Grade III or Grade IV with extension of contrast medium into epidural space suggestive of complete annular rupture
It has been shown that a Grade III or higher annular tear on modified Dallas classification is significantly associated with concordant pain reproduction on discography.[32] This pathoanatomical fact provides the rationale for current intradiscal therapies that utilize radiofrequency energy to thermally treat the painful annulus fibrosus disrupted by annular fissures. The mechanism of pain relief is still speculative. Arguably, the mechanism of action is via thermal destruction of nociceptors in the annulus; and, denaturation and contraction (modulation) of collagen, though neither has been proven to occur.[33-34]
Intradiscal Electrothermal Therapy (IDET; Smith and Nephew, Memphis, TN), Percutaneous Intradiscal Radiofrequency Thermocoagulation (PIRFT) using discTRODE™(Radionics, Burlington, MA) and Intradiscal Biacuplasty (IDB; Baylis Medical Inc., Montreal, Canada) are three current applications of this heating principle collectively called as Thermal Annular Procedures (TAP).
A. Intradiscal Electothermal Therapy (IDET)
IDET was first introduced by Saal and Saal in the late 1990’s. They reported favorable outcomes of pain reduction and functional improvement from 60-80% at the end of two years follow-up.[35] Subsequently, there were several clinical studies published with mixed clinical results. It can be argued that inappropriate selection criteria and lack of proper procedural technique may be responsible for the variable evidence of efficacy. IDET is indicated only for the treatment of internal disc disruption (IDD). Consequently, favorable outcomes are not expected if diagnostic criteria of IDD are not satisfied. The International Association for the Study of Pain (IASP), in its taxonomy, has elaborated the diagnostic criteria for IDD[36] as:
- No visible disc herniations seen on MRI or CT
- Concordant pain on provocation discography with negative controls
- Radial fissure is evident in the painful disc on post-discography computed tomography (CT)
To date, most observational studies of IDET reported favorable but limited outcomes. For discogenic pain with positive response to lumbar discography, two high-quality randomized controlled trials of IDET provided inconsistent evidence. Pauza et al.[37] demonstrated significant improvement in mean Visual Analog Pain Scale score (36% vs. 17%) and Oswestry Disability index (11% vs. 4%) among patients treated with IDET procedure compared to patients assigned to sham control. Overall, 56% of IDET group versus 38% of the sham group had a greater than 2 point improvement in their VAS. In addition, 24% of the IDET group versus 4% of the sham control had greater than 75% of pain relief. Approximately 40% of the patient treated with IDET obtained 50% pain relief in highly select patient population (though multilevel degenerative disc disease, a less favorable subgroup, was included). The numbers needed to treat, to achieve 75% pain relief, was five. It would not be unreasonable to argue for the fact that this intervention is worthwhile in a significant minority of strictly defined patient population to avoid surgery and its antecedent cost and complications. This study has been criticized for the strictness of their selection criteria which would potentially limit widespread application of its results in real clinical practice.
On the other hand, Freeman et al.[38] demonstrated no differences between IDET treated and sham control on pain and functional outcome measures. Though the selection criteria were less rigorous, this study may be reflective of true mix of the patients in real clinical practice. This study has been criticized for inappropriate selection of patients who are unlikely to benefit from IDET including patients with multilevel degenerative disc disease (≥ 3 levels of degenerated as evident on MRI),[39] reduced disc heights, patients receiving workers’ compensation claims,[40-41] and no mention of patients’ body habitus (body mass index).[42] This study was also criticized by the lack of placebo response which would be suggestive of methodological flaws in this study.
Helm et al.[43] in a systemic review published recently encompassing two randomized placebo-controlled trials (Pauza et al37 and Freeman et al.,[38] and sixteen observational studies concluded that IDET can offer significant symptom amelioration in one half of carefully selected patient population with chronic discogenic pain. Most recently, a position statement issued by North American Spine Society summarizing the best available evidence on the safety and efficacy of non-surgical interventional management of chronic low back pain concluded that “ for patients with less functional impairment, relatively well maintained disc heights, and discogenic pain caused by annular tears or protrusions less than 3-4 mm, IDET or another lesser invasive procedure would seem to be a reasonable first option if the patient is unwilling to tolerate his or her pain and disability.”[44] The indications and contraindications are listed below.[45]
Indications
- Axial low back pain of at least 6 months duration +/- leg pain. Axial pain > leg pain.
- Failure to respond to conservative management.
- History consistent with discogenic back pain.
- Positive concordant discogram at low pressure (< 50 psi above opening pressure) and negative control disc.
- One or two desiccated discs with or without small, contained herniated disc on MRI. Involved disc should have more than 50% residual disc height.
- At least Grade III annular tear such as radial or concentric fissures extending to the outer annulus fibrosus as evidenced on CT-discography.
Contraindications
Absolute Contraindications
In addition to the usual contraindications for any neuraxial interventions (such as systemic infection, local infection, coagulopathy, and patient refusal), the other ones that are particular to IDET include:
- Severe disc degeneration as evidenced by <50% of disc height on imaging
- Extruded or sequestered nucleus pulposus at affected level
- Previous lumbar back surgery (laminectomy, discectomy, or fusion) at the affected level
- IDET performed within last 6 months at the same level
- Results of MRI with evidence of nerve root compression, moderate to severe spinal canal stenosis, tumor or infection
- Moderate to severe end-plate degenerative changes (e.g., spondylosis) at the affected levels
- Grade I spondylolisthesis with motion on flexion/extension radiographs
- Grade II or greater spondylolisthesis
- Pregnancy
- Major psychologic impairment
Relative Contraindications
- Obesity[42]
- Workman Compensation claimants[41][46]
- Grade 1 spondylolisthesis with no or minimal motion on flexion/extension radiographs
- Smoking
Technique
Prerequisites
- Biplanar fluoroscopy
- IDET equipment including introducer (Trocar), navigable intradiscal catheter with embedded thermal-resistive coil and temperature sensor (SpineCath®, Smith and Nephew, Memphis, TN), IDET Generator
- Medications: Antibiotics prophylaxis against discitis, judicious use of short acting sedatives (level of sedation should be minimal to maintain meaningful communication with the patient)
- Gowns, drapes, solutions for sterile operative field
Patient Preparation
- Informed consent
- Standard NPO
- Patient position on fluoroscopy table in prone position
- Necessary monitors including electrocardiography, pulse oximetry and non-invasive blood pressure monitoring
Procedure
IDET is performed on the target disc preferably from the contra-lateral side of the annular disruption on the non-painful quadrant in a manner similar to lumbar provocation discography. The objective of the procedure is to deliver the distal and active, 5-cm tip of the navigable catheter across the outer half of the annulus on the painful side, as far posteriorly as possible, close to any circumferential lesion and across the radial fissure so that thermal lesion is produced in the outer innervated zone of annulus. For effective placement of the catheter, operator should review the CT-discogram prior to the procedure to determine the affected quadrant, and assess the location and depth of the annular/circumferential fissure. The technical aspects of the procedure are detailed below (Figure 1).
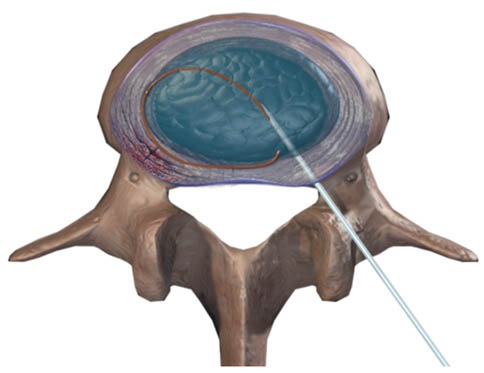
Figure 1. IDET: Intradiscal catheter is placed circumferentially
- As for any spinal procedure, the target disc is identified on AP fluoroscopy with perfect alignment of the vertebral endplates (“squared off view”).
- An oblique view of the target disc is obtained, where by superior articular process of the facet joint (SAP) of the target segment lies against the mid-point of the inferior end-plate.
- A puncture point is identified over the target point at the mid-height of target disc and ventral to the SAP projection. The introducer (Trocar) entry in a co-axial fashion at this insertion point should avoid the spinal nerve as it passes supero-lateral to this needle trajectory.
- After anesthetizing the needle trajectory, the 17-gauge trocar is introduced carefully in a co-axial fashion under oblique fluoroscopy until annulus is engaged (characterized by increase in resistance). At this point, patient should be warned of possibly an increase in back pain.
- Next, the trocar is advanced until its tip is at the centre of the disc on lateral view, and slightly medial to the pedicle on AP view.
- Flexible SpineCath is advanced through the trocar in-situ, and navigated to pass across the other side of the disc, then backwards, and eventually medially across the posterior annulus.
- Repeated AP/lateral views are recommended to ensure the safe passage of the catheter within the confines of annulus. The idea is to pass the catheter not too peripherally as to leave the annulus in the epidural space, and not too deep as to be remote from the outer innervated annulus (Figure 2-3).
- The circumferential nature of the path of the catheter can also be checked with a view known as “CT-view”, in which x-ray beam is directed upwards and forwards through the disc
- After confirmation of the position of the catheter, heating is commenced at 65°C and temperature is gradually increased up to final temperature of 90°C for a total of 16 to 17 minutes. Patient is warned at this stage as to the reproduction of his familiar pain during the heating process. However, pain radiating below the knee when performing the procedure at L4/L5 and L5/S1 or to the anterior thighs with L3/L4 procedures may be indicative of nerve root irritation and warrants readjustment of the probe.
- After completion of the heating protocol, the probe is removed and sterile bandage is applied at the insertion site
- Patient is held in observation and recovered from sedation.
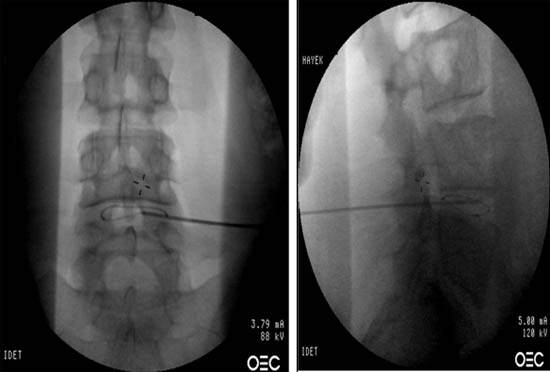
Figure 2. Fluoroscopic view of spineCATH intradiscal catheter inserted into L4-L5 disc. AP and lateral view.
Post-Procedural Care
- Patient is assessed for any possible complications and side effects in recovery room, which need to be addressed immediately.
- Patient may experience flare-up of their back pain for several weeks. Analgesics can be prescribed according to patient needs. Patients may use an ice pack on the site of the insertion the day of the procedure and warm moist heat the following day if they experience discomfort when the local anesthetic wears off.
- To avoid increased risk of re-injury from disc loading, lumbar corset to stabilize the spine is prescribed for several weeks to several months after procedure while healing of the outer annulus occurs.
- Limit sitting to 30 to 45 minutes at a time for the first six weeks.
- Return to activity varies on case by case. On average
- Sedentary activity at 1 to 3 weeks after the procedure
- Light duty at 6-8 weeks
- Moderate to medium duty at 12-16 weeks
- Driving is prohibited for first 5 days, then only 20 to 30 minutes at a time for the first six weeks.
- Riding as a passenger is acceptable for up to 45 minutes in a comfortable seat.
- Avoid bending, twisting or lifting > 10 pounds for the first 6 weeks.
- Walk 20 minutes daily after 1st week; advance to 20 minutes twice daily as tolerated.
- Do stretching exercises for legs (gently) after 1st week
- Avoid swimming in the 1st 6 weeks.
- Resume activity on a graded program, with attention to back care, commencing at approximately 8 weeks as tolerated, supervised by a physical therapist or physiatrist.
Complications
The complication rate in general appears to be low with the IDET procedure itself. Rather, the most common complications are those due to needle placement that arise from all intradiscal therapies:
- Transient paresthesias and exacerbation of back pain in the first several days are the most common complications requiring supplemental analgesics.
- Superficial skin infection
- Paraspinal abscess
- Discitis
Complications related to electrothermal treatment:
- Catheter kinking and breakage
- Cauda equina injury[47]
- Post IDET disc herniation[48]
- Vertebral osteonecrosis[49]
Clinical Pearls
- At any time during the procedure, if patient reports any sensation (radicular pain or burning foot), procedure should be stopped. Position of the trocar or catheter should be assessed using AP and lateral fluoroscopic views, and the trocar or catheter is repositioned as necessary.
- If catheter is not aligned close to the circumferential fissure, or the catheter is unable to cross the radial fissure, a two-stage procedure is initiated which entails the placement of second catheter from the opposite side, as close to the fissure as possible.
- If resistance is felt while advancing the catheter, avoid forcing it forward which may lead to catheter kinking or breakage. Rather, try to remove gently and reposition the coil. If resistance is still an issue, place another catheter from the opposite side after heating is finished from in-situ catheter
- Like all intradiscal procedures, multiplanar fluoroscopy should be used for confirmation of needle and coil placement. Sedation should be optimal to maintain meaningful communication between the operator and patient.
B. Percutaneous Intradiscal Radiofrequency Thermocoagulation (PIRFT)
Direct radiofrequency (RF) lesioning of intervertebral disc was first performed by Sluijter in 1988 with favorable early results. Earlier, randomized controlled trials of RF thermocoagulation of “intervertebral nucleus” failed to demonstrate significant pain relief and functional improvement.[50-51] Understandably, radiofrequency thermocoagulation applied by RF probe introduced to the center of a disc may not destroy any of the nociceptive fibers in the outer annular zone, the site of symptomatic disc disruption.
IDET, a thermal conduction source, converts the radiofrequency energy into conductive heat energy through its resistive wire-heating coil along the distal 5-cm of the catheter. Besides having the advantage of knowing the maximum temperature at the surface of the applicator to minimize the collateral damage, tissue penetration of such conductive source of energy is much less than the direct application of RF energy.[52] To overcome this inherent limitation of thermal conduction source, DiscTrode™ (Radionics Inc., Burlington, MA) was the first flexible RF electrode to provide “intra-annular” thermal therapy. As a direct RF catheter, it should provide more efficient heat transfer through the annulus and better pain relief. However, there is limited evidence available with regards to the efficacy of PIRFT using discTrode.[53-54] Interestingly, it seems to be clinically less effective than IDET for chronic discogenic pain.[55] Recently published randomized double-blind placebo controlled trial failed to demonstrate significant difference in pain relief and functional improvement between PIRFT with discTrode and sham PIRFT.[56]
Indications
It is the similar to IDET described above.
Contraindications
It is the similar to IDET described above.
Technique
Prerequisites
It is the same as for IDET mentioned above.
Patient Preparation
It is the same as for IDET mentioned above.
Procedure
- The discTrode™ introducer is directed to posterior lateral annulus contralaterally in a similar manner as that of IDET along with impedance monitoring and fluoroscopic guidance.
- Impedance monitor measures tissue resistance, detecting the higher resistance of the disc annulus with both sound and digital readings.
- A steerable radiofrequency electrode (discTrode™) is fed through the introducer and is navigated across the posterior annulus between the lamellae (Figure 3).
- An embedded temperature monitor is used in conjunction with external temperature monitor needle that can be placed into outer posterior annulus guided by impedance monitoring and fluoroscopy. It monitors the spread of thermal energy for safety during heating.
- Next, placement of the probe and external temperature sensor within the intervertebral disc annulus is confirmed using AP, lateral and oblique fluoroscopic imaging.
- Controlled heating is commenced beginning at 65°C but the final temperature is determined by measuring contralateral outer annulus temperature with external sensor.
- The temperature is maintained at 65°C for the next 4 minutes.
- Next, the probe is removed and sterile bandage is applied at the needle insertion site.
- Patient is held for observation and recovered from sedation.
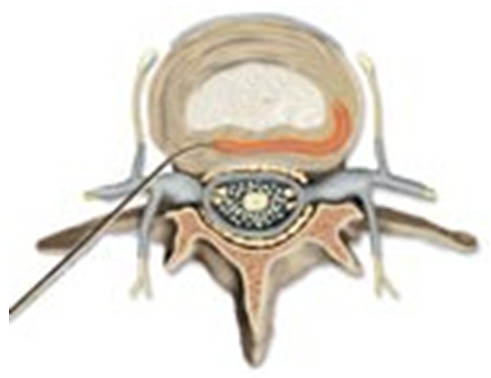
Figure 3. DiscTRODETM (flexible RF probe)
Post-Procedure Care
This is essentially same as that for IDET.
Complications
Discitis has been reported as a complication in one trial.[51] Potential PIRFT complications are similar to IDET.
Clinical Pearls
The depth of introducer insertion should be carefully monitored by electrical impedance values and lateral projection of fluoroscopic imaging to avoid the inadvertent entry into the central nucleus. Impedance values between 300 and 400 Ω usually corresponds to mid-annular position of the introducer when confirmed by lateral x-ray guidance. Additionally, from a safety standpoint, along with temperature monitoring with an external thermocouple sensor as discussed above, low voltage stimulation at 50 Hz should be carried out. Stimulation at < 0.5 V suggestive of electrode position in close vicinity of the nerve roots warrants repositioning.[54]
C. Intradiscal Biacuplasty (IDB)
The lack of universal success with IDET and PIRFT may be explained by the technical difficulties in appropriate placement of heating element and sub-optimal thermal distribution to the broader expanse of outer annulus resulting in insufficient denervation activity.[52][57] An ideal thermal annular procedure would be able to achieve adequate temperature profile (≥45°C) that is appropriate to induce neurolysis in the posterior disc annulus while maintaining safe temperatures (≤43°C) in the surrounding vital structures.[52][58] To potentially overcome these limitations, Intradiscal Biacuplasty using Trasndiscal™ system appears to be a promising addition to the armamentarium of thermal annular procedures.
IDB is a novel thermal annuloplasty technique employing an innovative internally-cooled radiofrequency system consisting of two radiofrequency probes placed in a bipolar arrangement. This bipolar Transdiscal™ system creates large controlled “strip” lesion across the posterior and postero-lateral annulus fibrosus. Preclinical studies on human cadavers demonstrated that a cooled radiofrequency system can achieve an improved heating profile (54±6°C to 60±6°C) while keeping temperatures in surrounding nerve root and epidural space safely below 45°C.[59-60] Also, relative ease of probe placement makes it an ideal substitute in a situation where placement of an IDET navigable catheter is challenging.[61] There is only one pilot study published with promising outcomes at the end of 12 months.[61-62]
Indications
They are similar to IDET as described above.
Contraindications
They are similar to IDET as described above.
Technique
Prerequisites
It is the same as for IDET mentioned above.
Patient Preparation
It is the same as for IDET mentioned above.
Procedure
- Two 17-gauge Transdiscal introducers are inserted percutaneously and directed to postero-lateral annulus bilaterally essentially in a same manner as that of IDET using fluoroscopy and optional impedance monitoring to ensure accurate placement.
- Two Transdiscal radiofrequency probes are then fed through each of the introducers to create a bipolar configuration (Figure 4).
- Next, placement of the probes within the intervertebral disc annulus is confirmed using AP, lateral and oblique fluoroscopic imaging.
- Next, probe temperatures are gradually increased to 45°C over 9 minutes. Subsequently, heating is maintained for 6 minutes at cooled electrode temperature of 45°C.
- The probes are removed and sterile bandages are applied on needle insertion sites
- Patient is held for observation and recovery from sedation
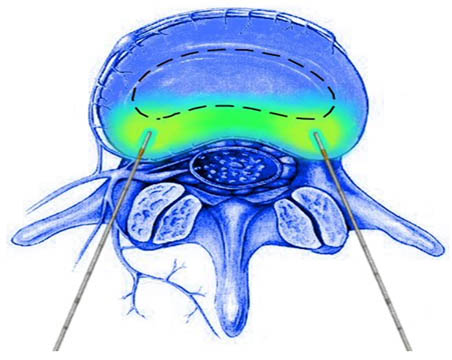
Figure 4. TransDiscal Bipolar arrangement. Current is concentrated between the two electrodes and creates a “strip” lesion across the posterior annulus fibrosus.
Post-Procedure Care
This is essentially the same as for IDET.
Complications
There are no reported complications specific to IDB that have been published thus far in the literature.
Clinical Pearls
To ensure more anterior-posterior parallel placement of trandiscal probes, less oblique angle is needed compared to provocation discography. Therefore, SAP is viewed at one-third of the disc width rather than in the middle of the disc.
Summary
The quest for successful, minimally invasive techniques for the treatment of chronic LBP continues to drive innovation in intradiscal techniques. Like in all of pain medicine, improved patient selection and robust, blinded clinical trials are needed to properly evaluate the efficacy of these techniques and establish their place in the paradigm of LBP treatment. The recent Center for Medicare and Medicaid Services (CMS) decision to deny coverage for IDET may hinder further development in the area of thermal annular procedures.
References
- Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA: Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009; 12: E35-E70
- Deyo RA, Mirza SK, Martin BI: Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976.) 2006; 31: 2724-7
- From the Centers for Disease Control and Prevention. Prevalence of disabilities and associated health conditions among adults--United States, 1999. JAMA 2001; 285: 1571-2
- Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA: Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997-2006. Spine (Phila Pa 1976) 2009; 34: 2077-84
- Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS: The rising prevalence of chronic low back pain. Arch Intern Med 2009; 169: 251-8
- Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, Buenaventura RM, Conn A, Datta S, Derby R, Falco FJ, Erhart S, Diwan S, Hayek SM, Helm S, Parr AT, Schultz DM, Smith HS, Wolfer LR, Hirsch JA: Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician 2009; 12: 699-802
- Shekelle PG, Markovich M, Louie R: An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976) 1995; 20: 1668-73
- Stanton TR, Henschke N, Maher CG, Refshauge KM, Latimer J, McAuley JH: After an episode of acute low back pain, recurrence is unpredictable and not as common as previously thought. Spine (Phila Pa 1976) 2008; 33: 2923-8
- Bogduk N: Clinical anatomy of the lumbar spine and sacrum, 4th edition. New York, Churchill Livingstone, 2005
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N: The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976) 1995; 20: 1878-83
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N: Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976) 1994; 19: 1132-7
- Maigne JY, Aivaliklis A, Pfefer F: Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976) 1996; 21: 1889-92
- Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI: Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997; 350: 178-81
- Burke JG, RW GW, Conhyea D, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM: Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine (Phila Pa 1976) 2003; 28: 2685-93
- Saal JA, Saal JS: Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Clin Sports Med 2002; 21: 167-87
- Hancock MJ, Maher CG, Latimer J, Spindler MF, McAuley JH, Laslett M, Bogduk N: Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J 2007; 16: 1539-50
- Wilke HJ, Neef P, Hinz B, Seidel H, Claes L: Intradiscal pressure together with anthropometric data - a data set for the validation of models. Clin Biomech (Bristol, Avon) 2001; 16: S111-S126
- Schmidt H, Kettler A, Rohlmann A, Claes L, Wilke HJ: The risk of disc prolapses with complex loading in different degrees of disc degeneration - a finite element analysis. Clin Biomech (Bristol, Avon) 2007; 22: 988-98
- Saifuddin A, Braithwaite I, White J, Taylor BA, Renton P: The value of lumbar spine magnetic resonance imaging in the demonstration of anular tears. Spine (Phila Pa 1976) 1998; 23: 453-7
- Aprill C, Bogduk N: High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol 1992; 65: 361-9
- Smith BM, Hurwitz EL, Solsberg D, Rubinstein D, Corenman DS, Dwyer AP, Kleiner J: Interobserver reliability of detecting lumbar intervertebral disc high-intensity zone on magnetic resonance imaging and association of high-intensity zone with pain and anular disruption. Spine (Phila Pa 1976) 1998; 23: 2074-80
- Carragee EJ, Paragioudakis SJ, Khurana S: 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976) 2000; 25: 2987-92
- Crock HV: Internal disc disruption. A challenge to disc prolapse fifty years on. Spine (Phila Pa 1976) 1986; 11: 650-3
- Bogduk N: Practice guidelines for spinal diagnostic and treatment procedures, 1st edition. San Francisco, International Spine Intervention Society, 2004
- Carragee EJ, Alamin TF, Carragee JM: Low-pressure positive Discography in subjects asymptomatic of significant low back pain illness. Spine (Phila Pa 1976) 2006; 31: 505-9
- Holt EP, Jr.: The question of lumbar discography. J Bone Joint Surg (Am) 1968; 50: 720-6
- Derby R, Lee SH, Kim BJ, Chen Y, Aprill C, Bogduk N: Pressure-controlled lumbar discography in volunteers without low back symptoms. Pain Med 2005; 6: 213-21
- Wolfer LR, Derby R, Lee JE, Lee SH: Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician 2008; 11: 513-38
- Bogduk N, Karasek M: Precision Diagnosis and Treatment of Back and Neck Pain. Continuum.American academy of neurology 2005; 11: 94-136
- Raj PP: Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract 2008; 8: 18-44
- Sachs BL, Vanharanta H, Spivey MA, Guyer RD, Videman T, Rashbaum RF, Johnson RG, Hochschuler SH, Mooney V: Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine (Phila Pa 1976) 1987; 12: 287-94
- Vanharanta H, Sachs BL, Spivey MA, Guyer RD, Hochschuler SH, Rashbaum RF, Johnson RG, Ohnmeiss D, Mooney V: The relationship of pain provocation to lumbar disc deterioration as seen by CT/discography. Spine (Phila Pa 1976) 1987; 12: 295-8
- Shah RV, Lutz GE, Lee J, Doty SB, Rodeo S: Intradiskal electrothermal therapy: a preliminary histologic study. Arch Phys Med Rehabil 2001; 82: 1230-7
- Smith HP, McWhorter JM, Challa VR: Radiofrequency neurolysis in a clinical model. Neuropathological correlation. J Neurosurg 1981; 55: 246-53
- Saal JA, Saal JS: Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine (Phila Pa 1976) 2002; 27: 966-73
- Merskey H, Bogduk N: Classification of Chronic Pain. Description of Chronic Pain Syndromes and Definitions of Pain Terms, 2 edition. Seattle, WA, IASP Press, 1994, pp 180-1
- Pauza KJ, Howell S, Dreyfuss P, Peloza JH, Dawson K, Bogduk N: A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J 2004; 4: 27-35
- Freeman BJ, Fraser RD, Cain CM, Hall DJ, Chapple DC: A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine (Phila Pa 1976) 2005; 30: 2369-77
- Kapural L, Mekhail N, Korunda Z, Basali A: Intradiscal thermal annuloplasty for the treatment of lumbar discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg 2004; 99: 472-6
- Mekhail N, Kapural L: Intradiscal thermal annuloplasty for discogenic pain: an outcome study. Pain Pract 2004; 4: 84-90
- Webster BS, Verma S, Pransky GS: Outcomes of workers' compensation claimants with low back pain undergoing intradiscal electrothermal therapy. Spine (Phila Pa 1976) 2004; 29: 435-41
- Cohen SP, Larkin T, Abdi S, Chang A, Stojanovic M: Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine (Phila Pa 1976) 2003; 28: 1142-7
- Helm S, Hayek SM, Benyamin RM, Manchikanti L: Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician 2009; 12: 207-32
- Derby R, Baker RM, Lee CH, Anderson PA: Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J 2008; 8: 80-95
- Kloth DS, Fenton DS, Andersson GB, Block JE: Intradiscal electrothermal therapy (IDET) for the treatment of discogenic low back pain: patient selection and indications for use. Pain Physician 2008; 11: 659-68
- Mekhail N, Kapural L: Intradiscal thermal annuloplasty for discogenic pain: an outcome study. Pain Pract 2004; 4: 84-90
- Hsia AW, Isaac K, Katz JS: Cauda equina syndrome from intradiscal electrothermal therapy. Neurology 2000; 55: 320
- Cohen SP, Larkin T, Polly DW, Jr.: A giant herniated disc following intradiscal electrothermal therapy. J.Spinal Discord Tech 2002; 15: 537-41
- Scholl BM, Theiss SM, Lopez-Ben R, Kraft M: Vertebral osteonecrosis related to intradiscal electrothermal therapy: a case report. Spine (Phila Pa 1976) 2003; 28: E161-E164
- Barendse GA, van Den Berg SG, Kessels AH, Weber WE, van KM: Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: lack of effect from a 90-second 70 C lesion. Spine (Phila Pa 1976) 2001; 26: 287-92
- Ercelen O, Bulutcu E, Oktenoglu T, Sasani M, Bozkus H, Cetin SA, Ozer F: Radiofrequency lesioning using two different time modalities for the treatment of lumbar discogenic pain: a randomized trial. Spine (Phila Pa 1976) 2003; 28: 1922-7
- Kleinstueck FS, Diederich CJ, Nau WH, Puttlitz CM, Smith JA, Bradford DS, Lotz JC: Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine (Phila Pa 1976) 2003; 28: 1700-8
- Erdine S, Yucel A, Celik M: Percutaneous annuloplasty in the treatment of discogenic pain: retrospective evaluation of one year follow-up. Agri 2004; 16: 41-7
- Finch PM, Price LM, Drummond PD: Radiofrequency heating of painful annular disruptions: one-year outcomes. J.Spinal Disord Tech 2005; 18: 6-13
- Kapural L, Hayek S, Malak O, Arrigain S, Mekhail N: Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med 2005; 6: 425-31
- Kvarstein G, Mawe L, Indahl A, Hol PK, Tennoe B, Digernes R, Stubhaug A, Tonnessen TI, Beivik H: A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy-a 12-month follow-up. Pain 2009; 145: 279-86
- Bogduk N, Lau P, Govind J, Karasek M: Intradiscal electrothermal therapy. Tech Reg Anesth Pain Manag 2005; 9: 25-34
- Haveman J, Sminia P, Wondergem J, van der ZJ, Hulshof MC: Effects of hyperthermia on the central nervous system: what was learnt from animal studies? Int J Hyperthermia 2005; 21: 473-87
- Kapural L, Mekhail N, Hicks D, Kapural M, Sloan S, Moghal N, Ross J, Petrinec D: Histological changes and temperature distribution studies of a novel bipolar radiofrequency heating system in degenerated and nondegenerated human cadaver lumbar discs. Pain Med 2008; 9: 68-75
- Pauza K: Cadaveric intervertebral disc temperature mapping during disc biacuplasty. Pain Physician 2008; 11: 669-76
- Kapural L, Ng A, Dalton J, Mascha E, Kapural M, de la GM, Mekhail N: Intervertebral disc biacuplasty for the treatment of lumbar discogenic pain: results of a six-month follow-up. Pain Med 2008; 9: 60-7
- Kapural L: Intervertebral disk cooled bipolar radiofrequency (intradiskal biacuplasty) for the treatment of lumbar diskogenic pain: a 12-month follow-up of the pilot study. Pain Med 2008; 9: 407-8
Leave a commentOrder by
Newest on top Oldest on top