Diabetic Neuropathy: A Problem-Based Learning Discussion
A 56-year-old man presented with a one-year history of fatigue, polyuria, polydipsia, blurred vision, and 7 kg weight loss. Physical examination revealed a BMI of 28 kg/m2 and reduced peripheral vibration and monofilament sensation on both feet. He was also complaining of a painful ‘pins and needles’ sensation to his feet up to his ankles.
Random blood glucose was elevated at 34 mmol/L and HbA1c was 140 mmol/mol (15.0%), confirming a diagnosis of DM. Basal-bolus insulin was instituted and resulted in resolution of hyperglycemia and osmotic symptoms. Insulin was subsequently discontinued, and he was discharged on metformin 1000 mg twice a day.
Three months later, the patient then complained of a new symptom of severe left-sided groin pain radiating down his left leg. There was associated left leg weakness, such that he walked with a quad cane. He went to the local ED. Examination revealed weakness of left ankle dorsiflexion and left knee extension, an absent left patellar reflex and an atrophied left quadriceps.
He was started on gabapentin and lumbar spine MRI was ordered which was negative for disk herniations, foraminal stenosis or other causes of lumbar plexopathy causing radiculopathy. Nerve conduction studies and electromyography (EMG) were ordered outpatient together with referral to PT.
HbA1c checked two months later returned at 42 mmol/mol (6.0%), indicating a clinically meaningful improvement in glycemic control. Over time his pain resolved, and he discontinued the gabapentin.
One year later, he continued to walk with a cane due to ongoing leg weakness, but two years following initial presentation, he could walk unaided.
Questions
1. Given this patient's medical history and review of systems, what is the differential of his foot drop?
The top of this differential should include diabetic polyneuropathy. Given this patient’s history of newly diagnosed diabetes mellitus, a form of diabetic neuropathy is a highly likely etiology. Studies have shown diabetic neuropathy prevalence estimates of up to 50% in patients with type 2 diabetes.1 Most commonly, diabetic neuropathy presents with symptoms including numbness, tingling, pain, and weakness in a distal, symmetric stocking and glove distribution.1 However, it is important to remember that patients with diabetes can develop other patterns including amyotrophy, radiculoplexopathy, mononeuropathy, and autonomic neuropathy and may rarely exhibit motor symptoms.2 In this scenario, the patient exhibits marked sensorimotor involvement in a unilateral lower extremity distribution in the setting of elevated Hemoglobin A1C. His symptoms improved and resolved as he began treatment with gabapentin with tighter glucose control as evidenced by significantly improved Hemoglobin A1C. Response to treatment, as in this case, supports an accurate diagnosis.
When evaluating foot drop, it may be helpful to organize the differential based on origin from various levels of the nervous system including the spinal cord, lumbosacral plexus, nerve roots, peripheral nerves, neuromuscular junction, and muscles. In this case, central causes (ex: cord compression, transverse myelitis) are less likely due to lack of upper motor neuron findings such as hyperreflexia or upgoing Babinski reflex. Characterization of the neurological deficits is helpful when starting to localize a lesion within the lower motor neuron system. This patient is exhibiting unilateral sensorimotor involvement, with weakness of dorsiflexion and knee extension, loss of knee reflex, and groin pain radiating down the leg. Given that this pattern is suggestive of involvement of multiple nerves, a pure muscle etiology such as a myopathy or a neuromuscular junction etiology such as amyotrophic lateral sclerosis would be less likely. Diabetic amyotrophy is a consideration though it classically presents with bilateral symmetrical muscle weakness and polyneuropathy. Mononeuropathy multiplex is also on the differential but is less associated with diabetes. The differential of peripheral nerve disorders is broad and includes trauma, compression as in carpal tunnel syndrome, autoimmune diseases, hormonal imbalances including hypothyroidism, uremia, nutritional imbalances including vitamin B12 deficiency, infections like Lyme disease and varicella-zoster virus, and medications such as chemotherapy drugs.3 Overall, it is important to recognize that there may be multiple factors or etiologies contributing to a presentation. In this case, the patient may have initially had peripheral diabetic neuropathy which improved, followed by possible diabetic amyotrophy. We cannot rule out an occult disc herniation not visible on MRI. A careful history, focused examination, and comprehensive diagnostic studies should be conducted to elucidate the etiology of a suspected peripheral neuropathy. As in this case, targeting the underlying cause can lead to improvement and even resolution of symptoms.
2. What is the pathogenesis of this condition?
The pathogenesis of diabetic neuropathy is thought to be multifactorial and complex. It is thought that oxidative and inflammatory stress in the setting of metabolic dysfunction may disrupt the balance between nerve fiber damage and repair.4 This may be due to increased circulating lipids and glucose that participate in pathways that lead to decreased ATP activity, increased reactive oxygen species, and significant mitochondrial dysfunction, all of which result in neuronal apoptosis and axonal failure.5 Additionally, it is thought that advanced glycation end products (AGEs) play a role in nerve damage. Vascular and hormonal changes related to diabetes may also play a significant role in pathogenesis.5
Diabetes preferentially affects the peripheral nervous system, and studies have shown that patients with diabetic neuropathy may first have C fiber loss corresponding to new onset pain, burning, and dysesthesias, while later developing large fiber axonal loss that may correspond with numbness or loss of proprioception.5
As the unilateral lower extremity symptoms resolved with gabapentin and tighter glucose control, they are likely related to a diabetic neuropathy process. Patients with diabetes can develop patterns of neuropathy including mononeuropathy and radiculoplexopathy.2 These processes are thought to be related to peripheral nerve ischemic injury, compression, and other unknown factors.2
3. What are the first line medications to treat pain in this setting? What are second line medications?
The first line medications for pain related to diabetic neuropathy include serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine), tricyclic antidepressants (amitriptyline) or the α2δ ligands (pregabalin, gabapentin) [Figure 1].6 Of these, only pregabalin and duloxetine have been approved by the Food and Drug Administration for treatment of diabetic neuropathy.6 However, multiple studies have found robust support for the treatment of pain associated with diabetic neuropathy using the other agents mentioned above. 7,8,4 There is a relative lack of comparative effectiveness studies that would suggest the best choice of the medications listed. Many of these medications are associated with adverse effects and drug interactions, especially in the elderly. Therefore, initial treatment should be individually selected based on the patient’s comorbidities and consideration of medication adverse effects, drug interactions, and cost.5 As an example, gabapentin may be a good option for a patient who also has restless leg syndrome, while amitriptyline should be avoided in an elderly individual with cardiac disease.
If the initial first line agent was ineffective, it may be discontinued and an alternate medication with a different mechanism of action should be started; if the initial first line agent had only partial efficacy, a second first line agent from a different class can be added to the current therapy[Figure 1].9 Overall, a treatment should be considered as ineffective when it has been titrated to a dose shown to be effective, for a duration of approximately 12 weeks. If the patient develops intolerable adverse effects within that time, the treatment should be discontinued. Combination therapy has been shown to be modestly more effective than monotherapy.10 However, a single agent is usually trialed first due to the potential for adverse effects.
Topical treatments for symptomatic relief include isosorbide dinitrate spray, lidocaine patches, and capsaicin cream. Other treatments, such as alpha lipoic acid and acetyl-L-carnitine have been shown in some studies to lead to symptomatic improvement, but studies investigating long-term improvement with these agents are lacking. Notably, transcutaneous electrical nerve stimulation has shown some promise in treating painful diabetic neuropathy.6
The American Neurological Association and American Diabetes Association do not recommend the use of this class of medications in treating diabetic neuropathy due to risk of addiction, sedation, and other complications.4 Tight glycemic control has been shown to be modestly effective in preventing neuropathy in those with type 2 diabetes, but its role in the management of already-present pain associated with diabetic neuropathy is unclear with varying reports.4 It is thus recommended to simultaneously treat the diabetes and pursue pharmacological treatment in order to both address the underlying pathophysiology and target the symptoms.
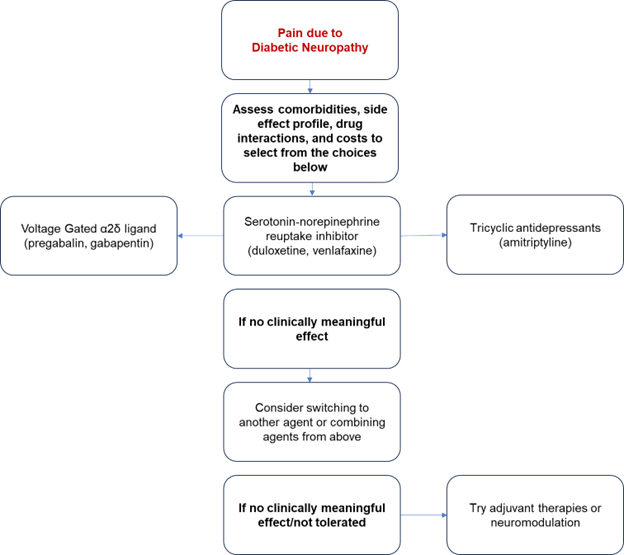
Figure 1: Visual Representation of the Diabetic Neuropathy Treatment Algorithm adapted from American Diabetes Association.4
In cases where diabetic amyotrophy is suspected, patients can be treated symptomatically as above, with management of hyperglycemia and pain management with options including NSAIDs and amitriptyline.11 Some studies have shown that early intervention with high-dose steroids can lead to more favorable outcomes in these patients, but there has yet been no conclusive evidence supporting use of immunomodulators in patients with diabetic amyotrophy.11
4. Your patient wants to try TENS on his leg for pain relief: is there any evidence for this?
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive therapeutic modality that has been used in the treatment of some individuals with peripheral diabetic neuropathy. The mechanism of TENS is essentially based on the gate control theory of pain and involves the use of cutaneous adhesive electrodes to stimulate A-beta fibers with the goal of indirectly inhibiting nociceptive transmission in the spinal cord dorsal horn. However, current evidence is weakened by a wide variety of devices and waveforms that technically fall under the umbrella term of “TENS,” thereby affecting the generalizability of results. Further, current literature shows mixed results, with some studies showing only minimal or even negative analgesic benefit. More research is warranted. However, it should be stated that TENS devices may still be a valuable adjunctive therapy for many patients due to their relatively low cost and high accessibility.12
5. What do you expect to find in this patient NCS and EMG?
Electrophysiologic testing allows to obtain measures of peripheral nerves and tributary muscles that are objective, independent of patient input, and unbiased. Usually when studying polyneuropathy of any cause, we need to include upper and lower limb peripheral nerves. Unilateral NCS are acceptable as the process of diabetic sensorimotor polyneuropathy is usually symmetric.13
The goals of doing NCS in these patients are to document the presence and severity of polyneuropathy by abnormal findings, document the extent of the disorder, and identify any changes that would suggest an alternative diagnosis. Differential diagnoses include radiculopathy, inflammatory myopathy, or motor neuronopathy, for which integration with EMG is needed.
Polyneuropathy associated with diabetes is characterized by the progressive loss of nerve fibers, both large and small.14 Since it is a length-dependent process that presents with sensory system dysfunction, it is important to screen for it by assessing a distal lower limb sensory nerve like the sural nerve. The evaluation of the sural nerve is thought to be more sensitive than the evaluation of other lower limb motor nerves.15 For motor nerve study of the lower limb, the peroneal and posterior tibial nerves can be used. As a general principle, if there are abnormalities seen on the initial screening, then an additional two sensory and two motor nerves should be tested. Also, if possible, nerves should be tested in three limbs in the more symptomatic areas.16
Normal values for sural nerve sensory testing with a foot superficial skin temperature of >30 degrees C are latency <4.2 ms, amplitude >5 μV. This is measured by placing the recording electrode at the posterior edge of the lateral malleolus, the reference on the anterior edge of the malleolus (ground can be placed on the Achilles tendon close to the bar electrode), and stimulating at 14 cm distal to the recording electrode between the heads of the gastrocnemius muscle. In the case of diabetic neuropathy, we will see decreased peak amplitude and reduced conduction velocity. Another candidate for sensory testing is the superficial branch of the fibular nerve.
In the peroneal motor nerve conduction studies, the compound muscle action potential can be elicited in three different locations: one eight cm above the line from the anterior ankle, one cm lateral to the tibial crest; another site at the fibular neck, below the head of the fibula; last site is at the lateral popliteal crease. It is normal to record a <10% decrease in amplitude with proximal stimulation (for example 10.2 mV with distal stimulation and 9.6 mV with proximal stimulation). Normative values are motor onset latency <6 ms, amplitude >2 mV, and conduction velocity >40. In this case, where the patient is experiencing weakness in ADF, the finding of a reduction in fibular CMAP amplitudes (ankle, below the lateral popliteal fossa or fibular head) would be compatible with axonal loss. When fibular neuropathy is present, F-wave responses (latency) at the affected side of the fibular neck may be prolonged or absent, while those on the unaffected side may be normal.
In the upper limb, median motor and sensory nerves together with the ulnar nerves are generally recommended for testing. Reference locations for sensory and motor testing are described in detail elsewhere.16 To note, a potential confounder to the diagnostic accuracy of NCS in this setting is that peripheral nerve function can be compromised in case of nerve compression resulting in NCS findings of mononeuropathy.17
It is important to note here that common limitations of NCS are relative to limb temperature and patient BMI. Cold extremities increase latency and high BMI makes location/identification/ proper nerve stimulation difficult.18 Both of these problems are commonly encountered in the chronic DM population. EMG is slightly more valuable to evaluate independent muscles but continues to be less reliable in the morbidly obese. For this reason, EMG for DM should include three to four limbs assessment to identify sensory pathology to verify a length-dependent sensory/motor polyneuropathy.
EMG has a limited diagnostic value in the diagnosis of diabetic polyneuropathy. In diabetic neuropathy, the EMG can show increased insertional activity, abnormal spontaneous activity (fibrillation potentials), and loss of recruitment, i.e., loss of the normal amount of motor unit potential activity, are observed in any process sufficiently impairing motor axons. Remodeling of motor unit potentials due to denervation with subsequent reinnervation is indicated by the presence of motor unit potentials with increased durations, increased amplitudes, and polyphasic form.
These are unfortunately nonspecific changes of an axonal process with the involvement of motor fibers and may be observed in different polyneuropathies as well as radiculopathies.
6. What is the relationship between HBA1C and painful diabetic neuropathy?
While up to 50% of diabetic neuropathy cases may have decreased sensation, including pain, others develop pain and dysesthesia induced by the involvement of small-diameter nerve fibers, which can be severe, often resulting in insomnia, mood disorders and poor quality of life. More recent studies show that neuropathic pain is present in 8.7% of impaired glucose tolerance (IGT) patients compared to 1.2% of those with normoglycemia.19
Plasma-glycosylated hemoglobin (HbA1c) is an indicator of metabolic control in diabetic patients, and it has been correlated with the severity of neuropathy. HbA1c levels provide an indication of average blood glucose concentrations during the preceding two to three months.20 The results of large-scale meta-analyses suggest that tight glucose control via HbA1c normalization can reduce the frequency of DPN in patients with type 1 diabetes (T1D) but has little or no effect on DPN in T2D.1 The large EURODIAB study followed prospectively 1,172 patients with type 1 DM and found that the cumulative incidence of neuropathy was related to the glycosylated hemoglobin value and the duration of diabetes, but also to other factors like high body mass index, elevated triglycerides, and smoking among others.21 It is still unclear if these findings relate directly also to type 2 DM, but it seems likely from multiple trials.22
The severity of disease can be suspected by the degree of abnormality of the NCS parameters; in the most severe cases, the motor and sensory responses are lost distally in the lower limb, and diffuse abnormalities in upper limb parameters are observed. To note, NCS can be misleading in case of mainly small fibers neuropathy since small fibers travel too slowly, and their conduction responses cannot be captured by a nerve conduction study. In these extreme cases a skin biopsy or QSART can be used.23,24
7. What are the interventional techniques that can be used to treat this type of pain?
While pharmacological approaches have been the mainstay of symptom management in patients with peripheral diabetic neuropathy-related pain, there are instances when medications are ineffective and non-traditional treatments might be warranted. In such cases, a growing number of interventional treatment options have become available and continue to develop including spinal cord stimulation (SCS), peripheral nerve stimulation (PNS), acupuncture, Botulinum toxin A (BTXA) injections, sympathetic nerve blocks, and surgical decompression of specific peripheral nerves among others.25 These modalities have shown promise to improve clinical outcomes of PDN and to decrease the use of drugs and their associated adverse effects.
A 2022 systematic review by Xu et al. sought to review recent advances in interventional therapies for PDN in order to evaluate the evidence—or lack thereof—and provide evidence-based recommendations in managing patients with refractory peripheral diabetic neuropathy. These researchers found that there is moderate to strong evidence to support the use of dorsal column spinal cord stimulation (SCS) in treating PDN in the lower extremities (Evidence level: 1B+), while studies investigating its efficacy in the upper extremities are lacking. Further, they found evidence to suggest that acupuncture and injection of Botulinum toxin-A provide relief in pain or muscle cramps due to PDN with minimal side effects (2B+/1B+). A similar level of evidence showed that surgical decompression of lower limb peripheral nerves was effective in patients with intractable PDN and superimposed nerve compression (2B±/ 1B+). Finally, in this review, Xu et al. stated that evidence for sympathetic blocks or neurolysis and dorsal root ganglion (DRG) stimulation was limited to case series (2C+) at the time that their study was performed.26
References
1. Feldman EL, Callaghan BC, Pop-Busui R, et al. Nat Rev Dis Primers 2019;5(41). https://doi.org/10.1038/s41572-019-0092-1
2. Tracy JA, Dyck PJ. The spectrum of diabetic neuropathies. Phys Med Rehabil Clin N A 2008;19(1):1-26. https://doi.org/10.1016/j.pmr.2007.10.010
3. Bodman MA, Varacallo M. Peripheral diabetic neuropathy. StatPearls [Internet]. 2023 https://www.ncbi.nlm.nih.gov/books/NBK442009. Published September 4, 2023. Accessed November 13, 2023.
4. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40(1):136-54. https://doi.org/10.2337/dc16-2042
5. Pop-Busui R, Ang L, Boulton AJM, et al. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy. Arlington, VA: American Diabetes Association; 2022.
6. Lindsay TJ, Rodgers BC, Savath V, et al. Treating diabetic peripheral neuropathic pain. Am Fam Physician 2010;82(2):151-58.
7. Bril V, England JD, Franklin GM, et al. Evidence‐based guideline: Treatment of painful diabetic neuropathy—report of the American Association of Neuromuscular and Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine & Rehabilitation. Muscle Nerve 2011;43(6):910-17. https://doi.org/10.1212/WNL.0b013e3182166ebe
8. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14(2):162-73. https://doi.org/10.1016/S1474-4422(14)70251-0
9. Price R, Smith D, Franklin G, et al. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN Guideline Subcommittee. Neurology 2022;98(1);31-43. https://doi.org/10.1212/WNL.0000000000013038
10. Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet 2022;400(10353): 680-90. https://doi.org/10.1016/S0140-6736(22)01472-6
11. Diaz LA, Gupta V. Diabetic amyotrophy. StatPearls [Internet]. 2023 https://www.ncbi.nlm.nih.gov/books/NBK560491. Published August 14, 2023. Accessed November 13, 2023.
12. Wang EJ, Berninger LE, Komargodski O, et al. Painful diabetic neuropathy-spinal cord stimulation, peripheral nerve stimulation, transcutaneous electrical nerve stimulation, and scrambler therapy: A narrative review. Pain Physician 2022;25(8):E1163-73
13. Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve 2002;25(2):212-17. https://doi.org/10.1002/mus.10044
14. Dyck, PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. Journal of Neuropathology & Experimental Neurology 1996;55(12),1181-93. https://doi.org/10.1097/00005072-199612000-00001
15. Kayser-Gatchalian MC, Neundörfer B. Sural nerve conduction in mild polyneuropathy. J Neurol 1984;231(3):122-25. https://doi.org/10.1007/BF00313678
16. Johnson EW, Pease, WS. Practical Electromyography. Baltimore, MD: Williams & Wilkins; 1988.
17. Perkins BA, Olaleye D, Bril V. Carpal tunnel syndrome in patients with diabetic polyneuropathy. Diabetes Care 2002;25(3):565-69. https://doi.org/10.2337/diacare.25.3.565
18. Miscio G, Guastamacchia G, Brunani A, et al. (2005). Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst 2005;10(4):354-58. https://doi.org/10.1111/j.1085-9489.2005.00047.x
19. Ziegler D, Rathmann W, Dickhaus T, et al. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg Surveys S2 and S3. Pain Med 2009;10(2):393-400. https://doi.org/10.1111/j.1526-4637.2008.00555.x
20. American Diabetes Association. Postprandial blood glucose. Clin Diabetes 2001;19(3):127-130. https://doi.org/10.2337/diaclin.19.3.127
21. Tesfaye S, Chaturvedi N, Eaton SE. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352(4):341-50. https://doi.org/10.1056/NEJMoa032782
22. Ang L, Jaiswal M, Martin C, et al. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep 2014;14(9):528. https://doi.org/10.1007/s11892-014-0528-7
23. Herrmann D, Griffin J, Hauer P, et al. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 1999;53(8): 1634-40. https://doi.org/10.1212/wnl.53.8.1634
24. Tobin K, Giuliani MJ, Lacomis D. Comparison of different modalities for detection of small fiber neuropathy. Clin Neurophysiol 1999;110(11):1909-12. https://doi.org/10.1016/s1388-2457(99)00164-9
25. Van Maurik JFMM, van Hal M, van Eijk RPA, et al. Value of surgical decompression of compressed nerves in the lower extremity in patients with painful diabetic neuropathy: a randomized controlled trial. Plast Reconstr Surg 2014;134(2):325-32. https://doi.org/10.1097/PRS.0000000000000369
26. Xu L, Sun Z, Casserly E, et al. Advances in interventional therapies for painful diabetic neuropathy: a systematic review. Anesth Analg 2022;134(6):1215-28. https://doi.org/10.1213/ANE.0000000000005860