Approach to Spinal Cord Stimulation Programming: A Problem-Based Learning Discussion
Case
A 65-year-old man with a history of chronic low back pain with intense bilateral lower extremity radicular pain was referred to our pain clinic. His medical history was significant for hypertension, obesity, and hyperlipidemia. In 1995, he underwent an L3-L5 laminectomy and posterior interbody fusion (PLIF) for lumbar stenosis with grade 2 spondylolisthesis of L4-L5 as per Meyerding classification. Despite initial resolution of his symptoms, he progressively had increasing back pain with radiation to the gluteal area bilaterally. The pain was constant and increased with activity, especially bending, and twisting his back. As a result of his debilitating pain, the patient was forced to retire from work as a carpenter. After failing extensive conservative management, including multiple physical therapy (PT) sessions, sacral medial branch blocks (MBB) followed by radiofrequency ablation (RFA) for suspect sacroiliitis, the patient underwent placement on spinal cord stimulation (SCS) in 1999. Specifically, the placement was with an SCS system with a single quadripolar surgical paddle lead (Resume 2®, Medtronic) with a constant voltage (Itrel®, Medtronic) implantable pulse generator (IPG).
After the initial programming, the patient had coverage with paresthesia of his low back (left > right) with > 50% pain relief for several years. However, about 10+ years after SCS implantation, the patient suffered a fall after tripping on his carpet at home and landing on his sacrum. The patient immediately presented to the emergency department in which hardware failure or fractures was ruled out. The patient was subsequently discharged home with a short course of oxycodone. Unfortunately, after the fall, the patient increasingly experienced low back and buttock pain, which was not alleviated by the SCS. This pain was debilitating and thus prompted his visit to our clinic.
Upon initial evaluation, he described his low back pain as constant, aching, and radiating bilaterally to the top of his hips and down into his bilateral gluteal areas. At that visit, he reported the SCS system covered the low back and his left side partially but could not appreciate any benefit on his right side. He described the pain as throbbing and as a paradoxical pain that was “cold, freezing, yet burning” in quality. The pain averaged a six of ten on a visual analog scale (VAS) and did not improve by rest. He suffered from weekly exacerbations of “lightning bolt-like” electrical sensations that would radiate below his knees. Physical examination demonstrated well-healed surgical scars of thoracic area, mild bilateral tenderness to palpation of the bilateral sacroiliac areas, no significant tenderness to palpation of the lumbar paraspinal area, and full muscle strength.
After demonstrating that the system was intact, by means of an implant x-ray and review of the electrodes’ impedances, the patient’s constant voltage SCS system was reprogrammed in the clinic on multiple occasions. We systematically and sequentially tested at fixed pulse width and frequency (210 micro-seconds, 90 Hz) to represent the greatest number of stimulation possibilities, with different electrodes configurations, starting with monopolar configurations first. After a careful review of the patient’s reported sensation and paresthesia level in defining a multicolumn contact configuration, we were able to identify three programs that ultimately were successful in increasing his pain coverage.
Questions
1. Given this patient’s symptoms, was he indicated for SCS placement? What are the currently FDA approved indications for SCS?
The current FDA approved indications for SCS are for the management of chronic intractable pain in the trunk or limbs, either bilateral or unilateral, associated with the following: failed back surgery syndrome, Complex Regional Pain Syndrome (CRPS) Types I and II, intractable low back pain and leg pain. Associated conditions include radicular pain syndrome, radiculopathies resulting in pain secondary to failed back syndrome or herniated disc, epidural fibrosis, degenerative disc disease (herniated disc pain refractory to conservative and surgical interventions), arachnoiditis, and multiple back surgeries.1
Despite this patient’s spinal surgery, he began to develop significant bilateral radicular pain. After undergoing all conservative management options including physical therapy, medial branch blocks, and radio frequency ablation, his pain persisted. Given failed attempts with various methods of management, the patient had the approved FDA indications for SCS. Patient received significant relief from his symptoms for over ten years.
2. The patient had pain relief for 10+ years prior to his fall. What are the long-term outcomes of SCS compared to other interventions in Persistent Spinal Pain Syndrome type 2 (like PNS, RFA, etc.)?
In a comprehensive literature review performed to compare the effectiveness of SCS compared to other methods from least invasive to most such as PT, medications, RFA, ESI, and intrathecal pumps, it was found that SCS had the most cost effective and longer-term pain relief as compared to the other modalities. Less invasive treatment options only provided temporary relief or required repeated injections for continued relief, whereas in patients with SCS, longer term relief was obtained with no need for repeat interventions.2
In a retrospective study done on 102 patients with SCS between 1989 and 2000, long term outcomes were studied along with associated complications and need for revision operations. Quigley et al. determined there were 64 revision operations done, which included replacement/repositioning, generator replacement, cable failure, and implant removal (five patients). It was reported that 68% had significant pain relief, despite revisions.3
In an alternate study done to determine the long-term outcomes of a closed-loop SCS, 50 patients had pain ratings using the visual analog scale, quality of life, function, sleep, and medication use were collected over 12 months. Russo et al. found that 81.4% had greater than 50% pain relief, and 53.5% had greater than 80% pain relief, and 84.9% of patients eliminated or reduced opioid intake.4
With these studies showing overall improvement of pain in patients with SCS, it can be recommended in patients who have refractory pain that have failed conservative management.
3. After the patient’s fall and presentation to the emergency department, his SCS was interrogated for acute complications. What are some common complications clinicians should be aware of?
There are several common complications of SCS that clinicians should be aware of including lack of effective pain control, device malfunction, and infection. Many studies have been conducted regarding the revision or removal of SCS systems.
The lack of effective pain control is cited as one of the most common reasons for revision and removal of SCS systems. In a retrospective study performed by Patel et al.,129 patients underwent removal of SCS due to lack of efficacy (80.6%), infection (14%), electrode migration (14%), and loss of stimulation (54%).5 Similarly, Rosenow et al. performed a retrospective study in which 43.5% of 577 procedures in patients required revision or removal of SCS due to poor pain relief6. Therefore, lack of effective pain control is a common reason clinicians should be aware of regarding removal or revision of SCS.
Device malfunction is a complication that can result from lead migration subsequently leading to lack of effective pain control. In a retrospective study performed by Leplus et al., 116 patients underwent tonic SCS. The authors found that 61% of patients required revision due to hardware dysfunction (32%), lead migration (23%), and infection (18%). In addition, the authors found that the benefit of revision decreases with numbers of revisions. This study demonstrates the importance of imaging when there is a question of device malfunction as it must be ruled out.7
In terms of infection, clinicians and patients should be aware of identifying and ruling out this complication of SCS. In a retrospective review performed by Falowski et al., 3.11% of 6,615 patients developed an infection within one year of implantation. Despite this, they found infection rate decreased with age (p<0.0001) and prior infection was associated with SCS infection (p<0.0001).8 Similarly, Antonovich performed a retrospective study in 291 patients in which 89 patients required reoperation. The authors found younger age was associated with reoperation (p<0.001).9
From these studies, clinicians should educate patients on the risks and complications associated with SCS. Management of expectations and education can assist with the prevention and management of complications. Therefore, this awareness can prevent further complications and poor outcomes.
4. Reprogramming SCS systems can be individualized such as in this patient.
a. Describe the different components of stimulation delivery: importance of amplitude, frequency, and pulse width.
SCS systems consist of electrodes and a generator that deliver small electrical impulses to the spinal cord. As a result, the pain pathway is disrupted, and a patient’s pain level is reduced. These electrodes are called leads and are placed adjacent to the spinal cord either as percutaneous or paddle. The generator is placed under the skin and delivers the impulses through programming.10 During a trial, the generator is placed externally as an external pulse generator (EPG).11
During the implantation phase, the electrodes are tested to ensure proper function. Information garnered through the trial phase is used as a guide for contact selection, programming amplitude, pulse width, and frequency. Patients will have two to three programs to optimize their pain relief. It is important for patients and clinicians to be aware that the SCS will not eliminate pain but rather manage pain. If a patient has high activity concerns, five to six programs can be developed.11
In terms of contact selection, a patient’s anatomy, needs, and preference should be considered. SCS leads differ in length, width, shape, and number of contacts. Percutaneous leads are cylindrical and paddle leads are flat and rectangular and round. Typically, two percutaneous or one paddle leads are implanted depending on the patient’s pain location. The shape of the electrical field is determined by lead separation and modified by arrangement of the cathode and anode. With less separation, the electrical field is larger creating more paresthesia coverage. When programming, amplitude, pulse width, and frequency will be adjusted. Amplitude determines how intensely the stimulation will be felt.11 The basic unit of stimulation is the waveform or pulse. The pulse width is defined as the sustained delivery of a certain amount of current amplitude for a certain amount of time. For example, narrow pulse widths require high amplitudes to deliver impulses to activate a neuron.10,12 This parameter will determine how broadly the stimulation is felt.11 Frequency is defined as the number of impulses per second. In the past, SCS rates have ranged from 40-100Hz to produce paresthesia. The rate of impulses will influence how often a neuron fires an action potential. Therefore, higher frequency may induce axonal blocking.10,12 Frequency can be adjusted so that paresthesia is not felt, thus determining the strength of stimulation.11 All these parameters can be altered to individualize treatment to manage chronic pain.10-12
b. What are the symptoms of lead migration and how can this be evaluated?
Lead migration may manifest as loss of pain coverage or paresthesia. When lead migration is suspected, patients must be evaluated through plain radiographs. This is the most efficient way leads can be evaluated. Previous studies have reported on defining what meaningful lead migration is defined as. For example, lead migration can be defined as any displacement from its desired location or loss of pain coverage that is not responsive to reprogramming.13
Lead migration can occur during a SCS trial and after many years after implantation. In terms of lead migration during SCS trial, risk factors and proper techniques must be identified.13 In regards to after SCS implantation, West et al. performed a meta-analysis to report the rates of lead migration. The authors found the incidence in patients was about one in ten patients. Clinicians need to be aware of the possibility of lead migration to educate patients on symptoms and when to be evaluated.14
c. How can one evaluate contact impedance levels?
In terms of SCS, contact impedance is defined as the device’s resistors making an opposing force to the current traveling through the electrodes. Impedance will increase with higher amounts of voltage.15 In addition, impedance can provide information on the structural integrity of the SCS device. For example, if impedance is high in an electrode contact, it could represent a lead fracture. This is especially relevant in MRI-conditional devices that function with normal lead impedances. If impedances are abnormal, this may affect MRI conditionality. Therefore, it is important to evaluate impedances to determine device functionality.16
d. How does one create bipoles or guarding programs for tonic stimulation in SCS devices?
In an SCS lead, electrodes are either configured as cathodes or anodes, and electricity flows from the anode to the cathode so that usually programming is started in fibers close to the cathode to obtain the largest depolarization.17 The electrical field can be shaped by selecting an appropriate configuration of anodes and cathodes. Some of the most used configurations include bipoles (adjacent/simple) and guarded cathode. In adjacent bipole, the cathode and the anode are close together, with between electrode spacing <4mm, to stimulate preferentially deeper dorsal column fibers which can be useful in cases of stimulation for low back pain with radiculopathy.18 Conversely, in a simple dipole configuration, there is a wider space between electrodes (>4mm), which allows to create a stimulation field that is elongated and shallow within the dorsal columns. This type of configuration can be useful in case the goal is to stimulate an entire extremity for example. Ultimately, in a guarded cathode the cathode is between two anodes, and such a configuration is thought to produce maximal recruitment of dorsal columns, which can be used to cover the low back and one-leg radiculopathy.17,18
e. What is the relationship between frequency and paresthesia?
Frequency in spinal cord stimulators can be adjusted so paresthesias are not detectable.10 The frequency parameter modulates both the intensity and perception of the stimulation. Tonic stimulation is hypothesized to induce paresthesias via dorsal column activation. Sub-paresthesia stimulation is subthreshold for dorsal column fibers activation, but the underlying mechanism is unclear. With tonic programming, patients perceive discrete pulses at low frequencies, and these pulses coalesce into a smooth tingling sensation as the frequency approaches 100 Hz. With paresthesia-free settings, the onset of analgesia typically exhibits a delayed response, consistent with a “wash in” effect.19 As frequency increases in the tonic range, paresthesias are perceived more intensely, which may enhance analgesia in the covered area. However, patients may trade off slightly more pain for slightly less paresthesias, as the tingling sensation can cause discomfort such as during sleep or machinery operation.10,19
f. When and why would a clinician change pulse width?
If current stimulator settings are not providing adequate pain relief, changing the duration of each electrical impulse, or pulse width can significantly alter how the nerves respond to stimulation. Instances in which one should consider changing pulse width are if there is inadequate pain relief, adverse effects of discomfort, battery life conservation, and tolerance development.10
g. How can clinicians or patients better understand when to change batteries and/or leads?
The decision to change the battery most often depends on battery life. Rechargeable batteries can last for around ten years and non-rechargeable batteries may last around five years.20 Factors that influence battery life are stimulation settings and type of device. Signs of low battery often include indicator signals like an alert sound but can also present as a decrease in pain relief, inconsistent stimulations, or other alerts on the device’s control unit. Leads themselves may need to be replaced when they shift or become damaged leading to a change in stimulation.16
h. Why are more contacts advantageous in neuromodulation?
It has been shown by several theoretical models that SCS electrodes are primarily stimulating large dorsal root fibers as they enter the spinal cord, despite the dorsal column neurons being spatially closer to the contacts. It is important to differentiate the two contingents of fibers since the dorsal root usually holds fibers coming from one dermatome, while the dorsal columns hold fibers from multiple dermatomes.21 The highest preferential stimulation of dorsal root fibers is obtained by monopolar stimulation through a large cathode, while the dorsal column fibers are recruited preferentially with small contacts/contact spacing. Several authors have supported this evidence, with small contact spacing resulting in the largest paresthesia coverage due to the higher recruitment of dorsal column fibers.22
i. What are the advantages of newer SCS systems in patients’ treatment?
The most relevant innovations in SCS systems in the last two decades can be grouped in lead(s) design, stimulation waveforms, and the advent of closed-loop technology.
Regarding leads design, while the initial hardware was inherited from cardiac pacemakers with inevitable complications linked to large electrode spacing, and rigid material with the potential for dural breaching, the field has moved towards increasing the number of contacts on a single lead wire (from one to four around the 1980s) until the more recent paddle electrodes with up to 16 contacts on the epidural side. This is a field with active ongoing research on novel polymers and electrodes coatings that minimize chronic inflammation in the host.23
The need for novel stimulation paradigms stems from the results of several randomized trials which confirmed the benefit of SCS, but also demonstrated that about half of the patients only had about 50% pain relief, and even the efficacy of pain relief could wane or be lost over time. This observation led to the development of stimulation paradigms that were different from the traditional tonic SCS which entails continuous stimulation at 40 to 100Hz. The high frequency stimulation, initially deployed at 10kHz with 30μs pulses with active recharge, and no paresthesia, has shown encouraging results in patients with refractory pain which were non-responders to tonic stimulation otherwise. Another paresthesia-free waveform is burst stimulation, which in its most common form uses a constant current stimulation at 500Hz with 1ms pulse width for periods of 10ms, whose starts are spaced 25ms apart. Several randomized placebo-controlled trials have shown burst stimulation is noninferior to tonic stimulation and may be better for certain aspects of pain.24 Multiple recent devices are now also capable of cycling or overlaying different waveforms including combinations of paresthesia-causing waveforms and non-paresthesia causing waveforms.
Lastly, closed loop stimulation entails modulating the amplitude of stimulation in response to ECAPs recorded from the device. In a blinded, randomized controlled trial, this form of closed-loop stimulation improved pain control in 82% of patients compared with 60% in open-loop stimulation.25
All the above innovations in programming especially can only exists on the newer SCS devices (lasting eight to ten years), with even more recent models only being capable to do PTM and even more complex stimulation paradigms.26
5. The patient had an FDA approved indication of SCS. What are some non-FDA, challenging indications of SCS? What evidence exists on these conditions?
Challenging indications of SCS include non-FDA approved indications such as refractory angina, peripheral vascular disease (PVD), pelvic pain, and cranial neuralgia. Although there is evidence that these conditions can be treated with SCS, it should only be implemented in conditions with refractory pain. Several studies have been performed investigating these conditions, suggesting that SCS should be considered an option in management.27
Refractory angina pectoris is typically treated with lifestyle modification, medications, and interventional management. In refractory cases, SCS can be considered although non-FDA approved. Langford et al. performed an observational study with 31 patients. The authors found that SCS was successful in the patient population.28 Similarly, Kumar et al. found 30 of 39 patients with PVD who were treated with SCS had improvement of pain and oxygenation.29 Lastly, Sankarasubramanian et al. found that in patients with conditions such as pelvic pain and cranial neuralgia SCS can provide pain relief and an improvement in daily activity. More specifically, this study showed all participants reported significant improvement in pain intensity (p<0.001), pain-related functional impairment and disability (p<0.001), pain coping (p<0.03), sleep (p<0.02), and overall health (p<0.005).30
6. The patient had an SCS system with a single quadripolar surgical paddle lead (Resume 2®, Medtronic) with a constant voltage (Itrel®, Medtronic) implantable pulse generator (IPG). Describe the most common available hardware used in the US: IPG and electrodes, vendors, stimulation paradigms.
The three most common vendors for IPGs and Leads are Medtronic, Boston Scientific, and Abbott (formerly known as St. Jude Medical). Medtronic IPGs include IntellisTM and VantaTM for example. Boston Scientific has the Precision SpectraTM and Spectra WaverightTM. Abbott has the Proclaim XRTM. Common leads by Medtronic are the Resume 2 quadripolar surgical paddle leadTM. They also have percutaneous leads like VectrisTM and SpecifyTM. Boston Scientific offers leads like Precision MontageTM and WaveWriterTM. Abbott has a multi-column paddle lead which is called the Penta leadTM.
Other than different hardware, there are also different stimulation paradigms that can be tailored to the patient’s individual needs. The most common are conventional tonic stimulation, burst stimulation, and high or low frequency stimulation.11
7. The patient had his SCS placement within his lumbar spine. Which are the recommended targets by vertebral level for stimulation of different anatomical regions?
Spinal cord stimulation (SCS) involves the delivery of electrical current to spinal elements including dorsal column (DC) and dorsal root (DR) fibers via electrodes placed in the dorsal epidural space. Stimulation of the DC and DR fibers creates a tingling sensation called paresthesia, which is detected by the patient. Overlap of paresthesia with the patient’s painful area(s) has been considered necessary for clinical success. Although paresthesia is useful in delineating the effective body areas covered by the stimulation, it is important to note here that this is not always necessarily the case long term, as there are available paresthesia-free stimulation paradigms.31
In general, sensory information from light touch and vibration stimuli is signaled through primary afferent axons that form DRs as they enter the spinal cord and ascend toward the brain via the DCs. The DCs are organized topographically with axons from more rostral dermatomes entering the cord more laterally. Stimulation of DC fibers generates paresthesia of several dermatomes caudal to the level of the stimulating cathode. As a general principle, when the topography of pain spans several dermatomes, the most effective stimulation target is the DC with a medial/para-medial placed electrode. Conversely, if the target is the dermatome of a specific nerve root, an electrode placed on that nerve root (lateral position) might be the best solution.
The location of the stimulating cathode determines the anatomic coverage of stimulation. He et al. reported a certain overlap between the location of electrodes relative to the vertebral level and the area of the body being stimulated.32 Because nerve depolarization generally occurs near a cathode, the electrode is typically placed at the spinal level to stimulate the corresponding DC maximally. In clinical practice, if the goal is to stimulate the face (intractable migraines) or the occipital area, then the most distal stimulating electrode should be placed at C2, since the dermatomes for the greater occipital nerve (C2/3), lesser occipital nerve (C2), and great auricular nerve (C2/3) are in that region, while trying to avoid stimulation to the dermatomes of trigeminal division V3 or the transverse cervical nerve. If the target area for stimulation is instead the neck or the shoulder, the stimulating contacts should span from C2-C4 vertebral levels. Stimulation of the upper extremity, especially forearm to hand, can be achieved by positioning the stimulating electrodes on C4 to C7 vertebral levels. Similarly, vertebral levels T1-T4 are usually chosen when it is needed to cover the chest wall (e.g. refractory angina), although we need to also consider that especially in this location comparable outcomes can be obtained with dorsal root ganglion (DRG) stimulation.33 If the goal is to cover the abdomen, make sure to place contacts at T5-T6. The most used location for back pain and lower extremity pain is T7-T10. It is theoretically possible to reach lower dermatomes by stimulating at L1-L2 for pelvis and groin pain, at L5-S1 to cover the foot, and at S2-S4 to relieve pelvic pain, but in clinical practice, the efficacy in these regions is not as robust as in the higher dermatomes.34
8. Troubleshooting is common for SCS such as in this patient. Describe a troubleshooting algorithm to use in the clinic for failure of previously working SCS systems.
Despite in general being considered a safe and reversible technology, the documented rate of SCS complications requiring surgical review is 30%-40%, with good patient selection currently being the most important factor influencing the outcome.35 Complications can be classified as hardware-related complications and biological complications. The former are more common than the latter, mainly those derived from problems with the electrodes, such as migration, which currently continues to be the most common complication. Lack of response is most related to a hardware problem, especially if this becomes noticed after six weeks or more from the surgery date. Figure 1 is a working diagnostic algorithm which can be used when troubleshooting an SCS device. To note, if at the end of the telemetry part there are no issues with impedances, it is necessary to consider reprogramming the SCS device. The first step when altering a previously working SCS contact’s configuration is to perform a ‘monopolar review’ by testing each individual contact as the (stimulating) cathode at different amplitudes with fixed pulse width and frequency to assess for location and perceived paresthesia by the patient. This intermediate step can further inform further programming of the SCS device to reach optimal coverage.
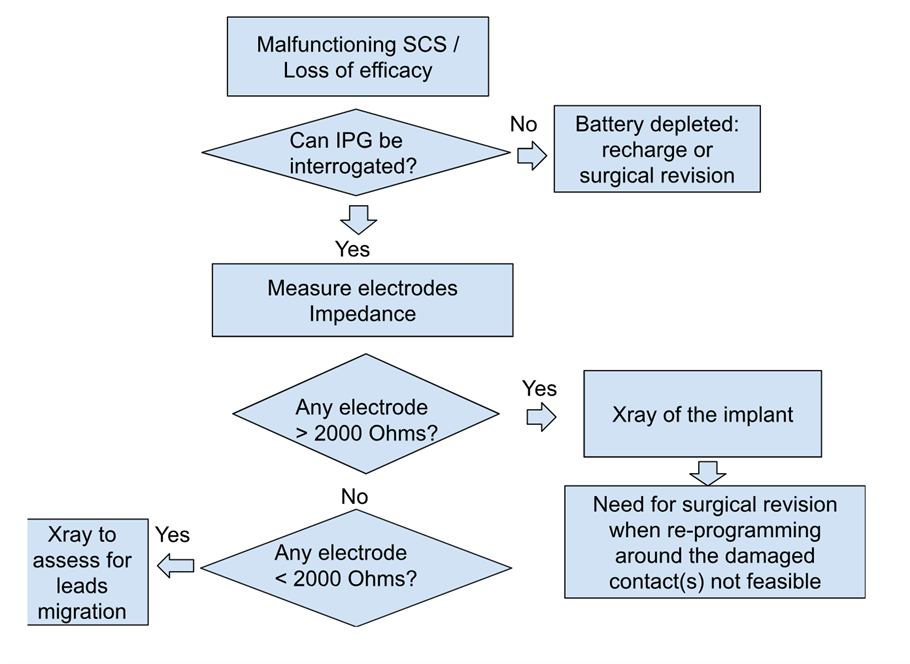
Figure 1: Visual algorithm for spinal cord stimulator troubleshooting.
References