Curb Your Enthusiasm: Local Anesthetic Adjuvants for Peripheral Nerve Blocks
Cite as: Ranganath YS, Seering MS, Marian AA. Curb your enthusiasm: local anesthetic adjuvants for peripheral nerve blocks. ASRA News 2020;45. https://doi.org/10.52211/asra110120.071
Regional anesthesia has become the cornerstone of perioperative pain management in modern surgical practice. The use of peripheral nerve blocks (PNBs) has become more widespread in recent years due to higher patient satisfaction, decreased opioid consumption and associated reduction in side effects, early hospital discharge, and advances in ultrasound technology.[1] For decades, extending the duration of sensory block for analgesia has been an appealing idea for regional anesthesiologists. Commonly used strategies include continuous catheter techniques, perineural adjuvants, and sustained-release local anesthetic (LA) molecules.[2]
There is a growing demand for finding a reliable solution for prolonging the duration of analgesia from PNBs
Intensity and the duration of postoperative pain are two major factors that determine the type of intervention necessary for optimal postoperative pain control. Single-injection PNBs are an attractive option because they are technically easier and can be quickly performed. In addition, they are less labor- and resource-intensive. They are widely used and are sufficient for a variety of procedures in which the pain intensity is high initially but is expected to diminish significantly over time. This allows for oral analgesics to be used effectively when the sensory and motor effects of the PNB are gradually subsiding. Pain management in some patients (opioid-tolerant patients, chronic pain patients) and following certain procedures (shoulder arthroplasty, ankle arthrodesis, limb amputations) is often challenging. Also, patients could suffer from rebound pain after the effect of the single-shot PNB wears off. [3] Therefore, there is a growing demand for finding a reliable solution for prolonging the duration of analgesia from PNBs. Continuous catheter techniques are widely employed to prolong regional analgesia; however, they have several drawbacks (summarized in Figure 1).
Continuous catheter techniques: Drawbacks
- Resource- and labor-intensive: Equipment cost, infrastructure setup, provider training, patient follow-up, and management until catheter removal
- Possibly longer procedure time and greater proficiency required
- Secondary block failure (19–26%),[4] catheter dislodgement, pump malfunction—these may render benefits intangible despite additional efforts.
- Multimodal analgesia and single-injection PNBs with adjuvants may be as effective.
Continuous catheter techniques: Benefits
- Superior analgesia up to 48 hours after orthopedic surgery[2; 5; 6]
- Earlier resumption of rehabilitation and physiotherapy with smoother transition to recovery[2; 5; 7]
- May allow some surgeries to be performed as outpatient/ambulatory procedures, reducing costs[8]
Figure 1: Drawbacks and benefits of continuous catheter techniques
Another common approach is to use adjuvants to prolong single-injection PNBs. A variety of drugs such as dexamethasone, dexmedetomidine, clonidine, opioids, epinephrine, magnesium, midazolam, and tramadol have been used as adjuvants with both neuraxial techniques and PNBs. [9; 10] This article will be restricted to the use of adjuvants with PNBs. Notably, a huge number of randomized controlled clinical trials (RCTs), along with systematic reviews and meta-analyses (SRMAs) (Table 1), have provided quality evidence regarding the use of perineural adjuvants. Despite this, there is not yet a clear answer as to whether the use of a perineural adjuvant is clinically superior to placebo or systemic administration of the same adjuvant drug.
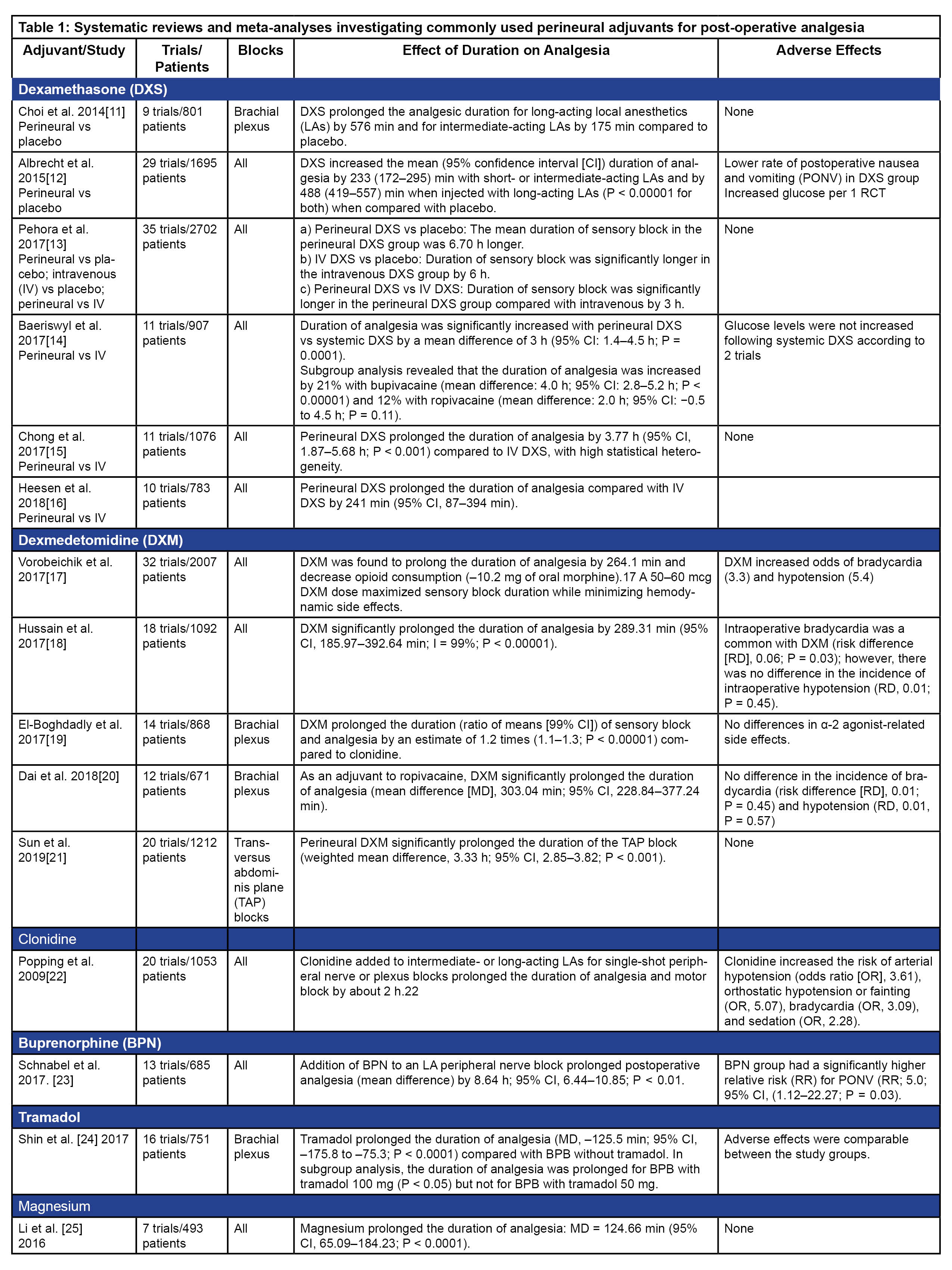
Summary of SRMA Information
Here we briefly review some of the relevant attributes for commonly used adjuvants before discussing their inconsistencies.
Dexamethasone: Dexamethasone is a potent synthetic corticosteroid with anti-inflammatory effects that has been studied extensively for its role as an adjuvant to LAs. Several mechanisms of action have been reported for its effect on the peripheral nerves, including inhibition of potassium channel-mediated discharge from nociceptive C fibers, reduction of ectopic neuronal discharge, and attenuation of release of inflammatory mediators. Dose:1–10 mg; 1 mg to 4 mg combined with ropivacaine for interscalene block prolonged the duration of analgesia in a dose-dependent manner; 4 mg represented a ceiling dose for prolongation of analgesia in a SRMA. Effect on analgesia: Compared to placebo, duration of analgesia is prolonged for long-acting LAs by 6–8 hours (intermediate-acting LAs by 3–4 hours). Prolongation of analgesia compared to systemic administration of dexamethasone is controversial—probably by 3–4 hours. Adverse effects: May increase blood sugar levels.
Dexmedetomidine: Dexmedetomidine, a selective α-2 adrenergic receptor agonist is another commonly used perineural adjuvant. Dexmedetomidine produces it effects through centrally mediated analgesia, α2-mediated vasoconstrictive effects, attenuation of the inflammatory response, and direct action on peripheral nerve-enhanced activity-dependent hyperpolarization in C fibers. Dose: 0.5–1.5 mcg/kg; 50–150 mcg; 50–60 mcg dose maximized sensory block duration while minimizing hemodynamic side-effects in an SRMA. Effect on analgesia: Compared to placebo, analgesia is prolonged by 4–5 hours. Adverse effects: Bradycardia and hypotension.
Clonidine: Clonidine is another α adrenergic receptor agonist used as a perineural adjuvant. Clonidine utilization has decreased significantly as dexmedetomidine (more selective for the alpha 2 receptor) has become more available and affordable. Dose: 30–300 mcg; 150 mcg is the commonly used dose. Effect on analgesia: Prolongs duration of analgesia by about 2 hours. Adverse effects: Bradycardia and hypotension.
Buprenorphine: Buprenorphine is a highly lipophilic partial opioid receptor agonist with LA properties. Dose: 100–300 mcg; 200 mcg or higher doses have been shown to be more effective in retrospective reviews. Effect on analgesia: Addition of buprenorphine to a LA peripheral nerve block prolongs postoperative analgesia for 8 hours. Adverse effects: Risk of postoperative nausea and vomiting (PONV) is significantly (5-fold) increased.
Others: Tramadol is a synthetic analog of codeine with noradrenergic, serotonergic, opioid agonist (mu), and peripheral LA effects. Addition of 100 mg of tramadol to a PNB-prolonged postoperative analgesia for 2 hours in an SRMA.[24] Magnesium is a NMDA (N-methyl-D-aspartate receptor) antagonist and was found to increase the duration of analgesia by 2 hours in another SRMA. [25] Several other drugs such as epinephrine, fentanyl, morphine, neostigmine, and midazolam have been evaluated as perineural adjuvants, but their routine use as adjuvants to long-acting LAs in PNBs is not recommended as they have not shown to significantly prolong the duration of analgesia. [9] It is to be noted that epinephrine is still commonly used in conjunction with LAs for PNBs either as a marker of intravascular injection or to prevent the higher peak in plasma concentration of LAs by delaying their systemic absorption.
Discussion
Given the vast amount of literature regarding the use of perineural adjuvants, it is surprising that there continues to be inconsistent findings reported in the literature. Although perineural dexamethasone has demonstrated efficacy in several SRMAs, there remains controversy that this finding may be secondary to systemic effects. [26; 27] In a recent study, addition of perineural or systemic dexamethasone to a low-volume (5 mL) interscalene block with 0.5% ropivacaine failed to produce clinically significant differences in outcomes between the two groups.[28] Multiple other clinical studies have demonstrated similar results. [29-31] Further, subgroup analysis in a previous SRMA demonstrated that perineural dexamethasone combined with bupivacaine, but not ropivacaine, prolongs the duration of analgesia when compared with systemic dexamethasone. [14] In addition, the quality of evidence supporting the use of perineural dexamethasone is graded as low to moderate by the authors of an SRMA. [26] These conclusions raise the probability that the effects of dexamethasone are more likely due to systemic absorption and anti-inflammatory effects, rather than as a result of direct effect on the nervous tissue.
Similarly, perineural dexmedetomidine and systemic dexmedetomidine (0.5 mcg/kg) prolonged the duration of analgesia to the same extent after brachial plexus blocks with ropivacaine. [32] Moreover, dexamethasone was found to be more effective than dexmedetomidine in an indirect meta-analysis where dexamethasone prolonged the duration of analgesia (no significant differences in sensory or motor block) by 2.5 hours more than dexmedetomidine. [33] Our own recent study failed to show benefits with any of the adjuvants (clonidine, dexamethasone and buprenorphine) added to LA for interscalene brachial plexus block. [34]
Several factors may be responsible for the discrepancies seen in clinical studies using perineural adjuvants. Firstly, the definition of duration of analgesia and the assessment protocols used in these trials were neither standardized nor consistent. Duration of analgesia varied from time to first analgesic request, to attaining a pain visual analogue score (VAS) of >3 or 4, or to the first patient self-report of postoperative pain at the surgical site. Secondly, crucial endpoints in these studies frequently occurred outside of the working hours of study personnel or after the patient was discharged from the hospital, which made accurate data collection difficult. Patient-reported outcomes used in some scenarios might be unreliable. Thirdly, the presence of surgical dressings, heterogeneity of surgical procedures, and scenarios in which the nerve block did not completely cover the surgical incision further complicate the assessment. Fourthly, most of the studies were small, with sample sizes of 10–50 patients/group, which increased the chances of type I error and publication bias. In addition, there were dosing variabilities. Lastly, many of these studies were performed in developing countries and published in low-impact journals, which could represent another source of publication bias. Funnel plots suggestive of high risk of publication bias are seen in meta-analyses.
Volunteer studies using perineural adjuvants have recently been utilized to address some of these concerns. In controlled volunteer studies, dexamethasone and clonidine have been found to be ineffective. Dexamethasone 4 mg (perineural and systemic) had no clinically relevant effect on the duration of sensory block provided by ropivacaine applied to the ulnar nerve in volunteers in a recent study. [35 Perineural clonidine added to ropivacaine in an adductor canal block volunteer study did not prolong the duration of sensory block in a set-up controlling for systemic effects of clonidine in volunteers. [36] Dexmedetomidine is the only drug that showed some effectiveness in volunteer studies. It has been studied in two volunteer studies with mixed results. One study revealed no clinically relevant prolongation of sensory blockade compared to systemic administration, and the other showed significant prolongation of sensory blockade. [37; 38 However, these volunteer studies did not take into account inflammation and other effects from surgery.
In addition, adjuvants may increase the incidence of side effects such as hypotension and bradycardia, [17; 18; 22] PONV, [23] and sedation. [22] The risk of neurotoxicity remains a concern for several adjuvants. [39] There is limited data evaluating the safety implications of perineural adjunct administration. Further, there is evidence that systemic administration of the adjuvant drug may result in similar prolongation of analgesic benefits when compared to perineural administration. This is an important consideration because there are no adjuvants currently approved by the FDA for perineural administration. Finally, the risk of drug errors, and subsequent neurotoxicity or adverse effects, when clinicians are compounding medications cannot be overlooked.
Conclusion
Even the most effective perineural adjuvants prolong analgesia by only a moderate duration and the maximum duration of effective pain control is limited to less than 24 hours following a single-injection PNB. Dexamethasone and dexmedetomidine are two of the most commonly used agents in current practice with dexmedetomidine administration likely producing more consistent results compared to dexamethasone. The low quality and clinical heterogeneity of published clinical trials, along with the inconsistent results, preclude any recommendation. Further, the risks and benefits associated with adjunct administration should be seriously considered given the potential risk of adverse effects, concerns for neurotoxicity, absence of benefits over systemic administration, and off-label use. Combined clinical and basic science research is necessary to enhance our understanding of the mechanisms of action and any undesirable effects (short- and long-term) of the adjuvants on peripheral nerves. Future research should seek to generate high-quality scientific evidence and focus on larger clinical trials designed to address some of the limitations discussed earlier. Despite their drawbacks, continuous catheter techniques may still represent a superior option in situations where prolonged regional analgesia is indicated.
Clinical Pearls
- Given the vast amount of literature in this area, there remains a significant amount of contradicting results.
- Similar prolongation of analgesia has been observed with systemic administration of adjunct drugs compared to perineural administration.
- Duration of analgesia following a single-injection PNB, even with most effective adjuvant, is limited to less than 24 hours.
- Recent volunteer studies have shown ineffectiveness of some of the commonly used adjuvants.
- More data does not necessarily mean good data. Methods to address factors responsible for inconsistent results should be considered in future studies.
References
- Albrecht E, Chin KJ. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia. 2020;75 Suppl 1:e101-e110.
- Ilfeld BM. Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124:308-35.
- Nobre LV, Cunha GP, Sousa PCCBd, Takeda A, Ferraro LHC. Peripheral nerve block and rebound pain: Literature review. Braz J Anesthesiol. 2019;69:587-93.
- Ahsan ZS, Carvalho B, Yao J. Incidence of failure of continuous peripheral nerve catheters for postoperative analgesia in upper extremity surgery.J Hand Surg Am. 2014;39:324-9.
- Bingham AE, Fu R, Horn JL, Abrahams MS. Continuous peripheral nerve block compared with single-injection peripheral nerve block: A systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2012;37:583-94.
- Vorobeichik L, Brull R, Bowry R, Laffey JG, Abdallah FW. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120:679-92.
- Malik T, Mass D, Cohn S. Postoperative analgesia in a prolonged continuous interscalene block versus single-shot block in outpatient arthroscopic rotator cuff repair: A prospective randomized study.Arthroscopy. 2016;32:1544-50.e1.
- Saporito A, Sturini E, Borgeat A, Aguirre J. The effect of continuous popliteal sciatic nerve block on unplanned postoperative visits and readmissions after foot surgery – a randomised, controlled study comparing day-care and inpatient management.Anaesthesia. 2014;69:1197-205.
- Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: A systematic qualitative review. PloS One 2015;10:e0137312.
- Prabhakar A, Lambert T, Kaye RJ, et al. Adjuvants in clinical regional anesthesia practice: A comprehensive review. Best Pract Res Clin Anaesthesiol. 2019;33:415-23.
- Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112:427-39.
- Albrecht E, Reynvoet M, Fournier N, Desmet M. Dose-response relationship of perineural dexamethasone for interscalene brachial plexus block: A randomised, controlled, triple-blind trial.Anaesthesia 2019;74:1001-8.
- Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. 2017;11:Cd011770.
- Baeriswyl M, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Efficacy of perineural vs systemic dexamethasone to prolong analgesia after peripheral nerve block: A systematic review and meta-analysis. British J Anaesth. 2017;119:183-91.
- Chong MA, Berbenetz NM, Lin C, Singh S. Perineural versus intravenous dexamethasone as an adjuvant for peripheral nerve blocks: A systematic review and meta-analysis. Reg Anesth Pain Med. 2017;42:319-26.
- Heesen M, Klimek M, Imberger G, Hoeks SE, Rossaint R, Straube S. Co-administration of dexamethasone with peripheral nerve block: Intravenous vs perineural application: Systematic review, meta-analysis, meta-regression and trial-sequential analysis. Br J Anaesth. 2018;120:212-27.
- Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: A systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118:167-81.
- Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: A systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42:184-96.
- El-Boghdadly K, Brull R, Sehmbi H, Abdallah FW. Perineural dexmedetomidine is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: A systematic review and meta-analysis. Anesth Analg. 2017;124:2008-20.
- Dai W, Tang M, He K. The effect and safety of dexmedetomidine added to ropivacaine in brachial plexus block: A meta-analysis of randomized controlled trials. Medicine. 2018;97:e12573.
- Sun Q, Liu S, Wu H, et al. Dexmedetomidine as an adjuvant to local anesthetics in transversus abdominis plane block: A systematic review and meta-analysis. Clin J Pain. 2019;35:375-84.
- Popping DM, Elia N, Marret E, Wenk M, Tramer MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: A meta-analysis of randomized trials. Anesthesiology 2009;111:406-15.
- Schnabel A, Reichl SU, Zahn PK, Pogatzki-Zahn EM, Meyer-Friessem CH. Efficacy and safety of buprenorphine in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2017;34:576-86.
- Shin HW, Ju BJ, Jang YK, You HS, Kang H, Park JY. Effect of tramadol as an adjuvant to local anesthetics for brachial plexus block: A systematic review and meta-analysis. PloS One.2017;12:e0184649.
- Li M, Jin S, Zhao X, et al. Does magnesium sulfate as an adjuvant of local anesthetics facilitate better effect of perineural nerve blocks?: A meta-analysis of randomized controlled trials. Clin J Pain.2016;32:1053-61.
- Marhofer P, Columb M, Hopkins PM. Perineural dexamethasone: The dilemma of systematic reviews and meta-analyses. Br J Anaesth. 2018;120:201-3.
- Hewson D, Bedforth N, McCartney C, Hardman J. Dexamethasone and peripheral nerve blocks: Back to basic (science). Br J Anaesth. 2019;122:411-2.
- McHardy PG, Singer O, Awad IT, et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: A randomised equivalence trial. Br J Anaesth. 2020;124:84-91.
- Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: A randomized, triple-arm, double-blind, placebo-controlled trial.Reg Anesth Pain Med. 2015;40:125-32.
- Desmet M, Braems H, Reynvoet M, et al. IV and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111:445-52.
- Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia. 2016;71:380-8.
- Abdallah FW, Dwyer T, Chan VW, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: A randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology.2016;124:683-95.
- Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block: Systematic review and indirect meta-analysis. Anesth Analg. 2019;128:543-54.
- Seering MS, Bayman EO, Wong CA, Ranganath YS, Marian AA. Comparison of the effect of three different adjuvants on the analgesic duration of single injection interscalene brachial plexus block: A prospective, randomized, triple blinded clinical trial.Reg Anesth Pain Med. Published Online First: 14 Jul 2019. doi: 10.1136/rapm-2018-100201.
- Marhofer P, Columb M, Hopkins PM, et al. Dexamethasone as an adjuvant for peripheral nerve blockade: A randomised, triple-blinded crossover study in volunteers. Br J Anaesth. 2019;122:525-31.
- Andersen JH, Jaeger P, Sonne TL, Dahl JB, Mathiesen O, Grevstad U. Clonidine used as a perineural adjuvant to ropivacaine, does not prolong the duration of sensory block when controlling for systemic effects: A paired, blinded, randomized trial in healthy volunteers. PloS One. 2017;12:e0181351.
- Andersen JH, Grevstad U, Siegel H, Dahl JB, Mathiesen O, Jaeger P. Does dexmedetomidine have a perineural mechanism of action when used as an adjuvant to ropivacaine?: A paired, blinded, randomized trial in healthy volunteers.Anesthesiology. 2017;126:66-73.
- Andersen JH, Jaeger P, Grevstad U, et al. Systemic dexmedetomidine is not as efficient as perineural dexmedetomidine in prolonging an ulnar nerve block. Reg Anesth Pain Med. Published Online First: 23 January 2019. doi: 10.1136/rapm-2018-100089.
- Knight JB, Schott NJ, Kentor ML, Williams BA. Neurotoxicity of common peripheral nerve block adjuvants. Curr Opin Anaesthesiol. 2015;28:598-604.
Leave a commentOrder by
Newest on top Oldest on top