Update for Healthcare Providers on the Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use
Joshua Romero, MD, and Christopher Ha, DO
Department of Physical Medicine & Rehabilitation, Mayo Clinic, Rochester, MN
Christine Hunt, DO, MS
Department of Anesthesiology & Perioperative Medicine, Division of Pain Medicine, Mayo Clinic, Rochester, MN
How Does the FDA Define “Humans Cells/Tissues (HCT/P)?”
- Human cells or tissues intended for implantation, transplantation, infusion, or transfer into the human body are collectively regulated by the U.S. Food and Drug Administration (FDA). These products are regulated as a human cell, tissue, and cellular and tissue-based product or HCT/P.
- The Center for Biologics Evaluation and Research (CBER) is one center within the FDA which is primarily in charge of regulating HCT/Ps and reviews new biologic products that come to market to ensure their safety and efficacy.
- Typically, biologic products are introduced through a Biologics Licensing Application and undergo a premarket review and approval process prior to marketing. There is a subset of products, however, that is exempt from the premarket approval process if they meet certain criteria and eligibility requirements.
How Are HCT/P Regulated?
- The FDA has released a series of guidance documents to help clarify the criteria that allow biologics to meet HCT/P designations as well as the qualifications for exclusion from the premarket approval process. In July 2020 the FDA released their most updated guidance memo regarding the criteria of “minimal manipulation” and “homologous use” for HCT/Ps to help improve stakeholders’ understanding of these key qualifications as part of the larger regulatory criteria surrounding HCT/Ps (flowchart on page 6).
- These regulatory criteria are not legally enforceable, but rather are recommendations unless specific regulatory or statutory requirements are cited.
- These guidance documents are also subject to changes and updates requiring regular review by researchers and clinicians engaged in patient care related to these products.
Updates on Minimal Manipulation
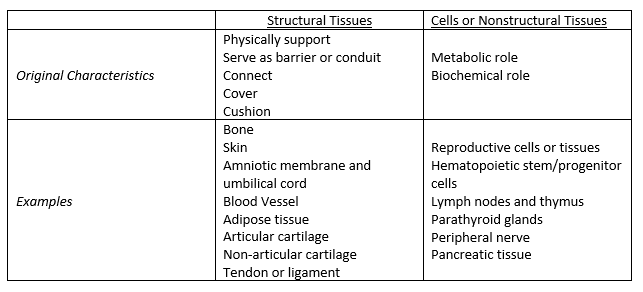
- Minimal manipulation is dependent on whether the HCT/P is a structural tissue or cell/nonstructural tissue.
- To be considered minimal manipulation for structural tissue, the processing of the HCT/P must not alter the original relevant characteristics of the tissue and its ability for reconstruction, repair, or replacement. Structural tissues include HCT/Ps that physically support, serve as barrier or conduit, connect, cover, or cushion. Examples include bone, skin, amniotic membrane, adipose tissue, tendon, and ligament. Specifically highlighting adipose tissue, this is considered minimally manipulated if its processing does not alter its ability to provide cushioning and support. Relevant characteristics include strength, flexibility, cushioning, covering, compressibility, and response to friction and shear.
- For cells or nonstructural tissue, the processing of the HCT/P must not alter the relevant biological consequences of the tissue. Cells or nonstructural tissues include HCT/P that have metabolic or biochemical roles in the body. Examples include reproductive cells or tissue, lymph nodes, hematopoietic stem cell/progenitor cells, and parathyroid glands (please see article for full list). Relevant characteristics include differentiation and activation state, proliferation potential, and metabolic activity.
- This newly released guidance document outlines original and relevant characteristics for both structural tissues and cells/nonstructural tissue (Table 1). It is important to understand how to differentiate structural tissues from cells/nonstructural tissues given the differences in safety and efficacy concerns between these two categories.
- For an HCT/P to be considered “minimally manipulated,” the original relevant characteristics must be unchanged.
- An example would include grinding bone to form bone chips as this does not alter its ability to support bodily structures. If the integrity of bone was altered as to no longer support its function to carry out these processes, it would no longer be considered minimally manipulated.
- When considering the removal of cells from a structural tissue, separation into its component parts must not alter the remaining structural tissues’ ability to reconstruct, repair, and/or replace homologous structures or function. This includes modification of an HCT/P’s physical state via mechanical or chemical means.
- For example, removing cells from adipose tissue that leave a decellularized extracellular matrix would be considered more than minimal manipulation because this alters its ability to provide cushion and support (original relevant characteristics).
- Storage should not alter the original characteristics of the tissue when placed in a buffer solution, tissue medium and refrigerated, or cryopreserved with liquid nitrogen vapor.
Table 1. Characteristics and examples of structural and nonstructural tissues.
Updates on Homologous Use
- Homologous use is an additional criterion that the FDA uses to determine if HCT/Ps require premarket approval. The definition for homologous use is the repair, reconstruction, replacement, or supplementation of a recipient’s cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor. HCT/Ps can act on cells or tissues that may or may not be identical to the donor cells or tissues, as long as it performs one or more of the same functions in the recipient as the cells or tissues originally performed in the donor.
- Basic functions are those features that are commonly attributed to the HCT/P as it exists in the donor. One example would be understanding that the cornea protects the eye and serves as its outermost lens. Structural tissues generally perform structural functions such as serving as a barrier, conduit, or covering, whereas cellular or nonstructural tissues usually have metabolic or biochemical functions, such as immune or endocrine functions. Those basic functions that the HCT/P is expected to perform in the recipient must be a basic function that it performed in the donor.
- One example provided in the guidance document is a heart valve that is transplanted to replace a dysfunctional heart valve. In this case, the recipient tissue is identical to the donor tissue (valve for valve) and performs the same basic function of ensuring unidirectional flow in the heart.
- A second example is pericardium used as a wound covering for dura mater defects. While the recipient dura mater is distinct from the donor pericardium, the pericardium is intended to serve as a covering in the recipient, which is one of the basic functions it performs in the donor.
- If an HCT/P is intended to be used as an unproven treatment for a myriad of diseases or conditions, it is likely not intended for homologous use only.
- An example would be infusing hematopoietic stem cells derived from bone marrow intravenously into a patient to treat cerebral palsy, presumably through the repair of brain tissue by differentiation into neuronal cells. This would not stand as homologous use given that there is not enough evidence to support that repair of neurologic tissue through differentiation into neuronal cells is a basic function of these cells in the donor.
What Is the New Guidance Surrounding Adipose Tissue?
- The July 2020 guidance document explicitly defines the recovery of adipose by liposuction and subsequent isolation of cellular components by enzymatic degradation or mechanical disruption to be considered more than minimal manipulation by the FDA.
- The adipocytes surrounding cells become disrupted by these processes and therefore the cushioning function of the adipose tissue would have been more than minimally manipulated.
- As such, adipose tissue undergoing mechanical or enzymatic degradation would require regulation beyond section 361 of the Public Health Service (PHS) Act and 21 Code of Federal Regulations (CFR) part 1271, requiring premarket approval in order to be used in a commercial or clinical setting outside of the context of FDA-regulated research.
- The basic function of adipose tissue includes providing cushioning and support for other tissues, storing energy in the form of lipids, and insulating the body. The use of an HCT/P from adipose tissue for the treatment of degenerative, inflammatory, or demyelinating disorders is considered to fall under the category of non-homologous use. This includes musculoskeletal conditions such as arthritis or tendonitis, which are commonly treated by the pain physician.
- The idea that an HCT/P from adipose tissue can regenerate or promote the regeneration of articular cartilage or tendon is not considered homologous use because it is not a basic function of adipose tissue.
- In the most recently released guidance document, this distinction is explicitly made: “An HCT/P from adipose tissue is used to treat musculoskeletal conditions such as arthritis or tendonitis by regenerating or promoting the regeneration of articular cartilage or tendon. This is generally not considered a homologous use because regenerating or promoting the regeneration of cartilage or tendon is not a basic function of adipose tissue.”
Scope of Regulation and Enforcement Policy
- The recommendations discussed so far apply to products and establishments defined in 21 CFR 1271. This does not apply to products outside of these definitions or to establishments that meet the same surgical exemption in 21 CFR 1271.3(d). For example, platelet rich plasma (PRP) falls outside the scope of this guidance as it is a blood product.
- The FDA intends to enforce discretion through May 31, 2021, as outlined in section V. Although not technically legally binding, these guidance documents may serve to inform clinicians and researchers of the FDA’s evolving thinking on the use of HCT/P and regenerative medicine products, and how the field may continue to develop along both trajectories.
Conclusion
- As the promise of regenerative medicine continues to grow, so does the need for a clear regulatory pathway to ensure the safety and efficacy of the products coming to market as well as those being used without the same regulatory oversight as products not meeting the criteria for exemption, as are increasingly used in clinical practice today.
- To overcome the moniker of the “Wild, Wild West,” it is incumbent upon those practicing in the field of regenerative medicine to remain up to date regarding their knowledge of the evolution of FDA guidance documents.
- The FDA has made it clear that with the expanding interest in the field, there is an increasing number of regenerative medicine products subject to FDA premarket authorization. It seems that additional scrutiny is being paid to the use of adipose tissue in regenerative medicine products, and mechanical degradation as well as use in musculoskeletal applications is now considered to violate the FDA’s criteria of minimal manipulation and homologous use, respectively.
- From a clinical perspective, understanding the concepts of minimal manipulation and homologous use helps practitioners maintain a science-based, patient-centered approach as they evaluate the credibility of new therapies that emerge in the field of regenerative medicine and scrutinize their own clinical and research practice.
Leave a commentOrder by
Newest on top Oldest on top