Neuraxial Hardware Infection: A Problem-Based Learning Discussion
A 64-year-old woman with a past medical history of type 2 diabetes mellitus (HgbA1C: 7.4) and hypertension was deemed to be a candidate for a percutaneous spinal cord stimulator (SCS) trial for Failed Back Surgery Syndrome. She had excellent results in the trial period which lasted 10 days and is now two weeks post-op from implantation. She calls your clinic with concerns of itching and redness around the site of the implanted battery. She presents to clinic with the following vitals: heart rate 80, blood pressure 130/84, respiratory rate 16, temperature 36.3 Celsius. Physical exam reveals the implanted pulse generator (IPG) incision site is erythematous, warm to the touch, and the erythematous region is tender to palpation (Figure 1). The patient denies systemic symptoms like fevers or chills, denies purulent drainage, and is ambulating well on her own.
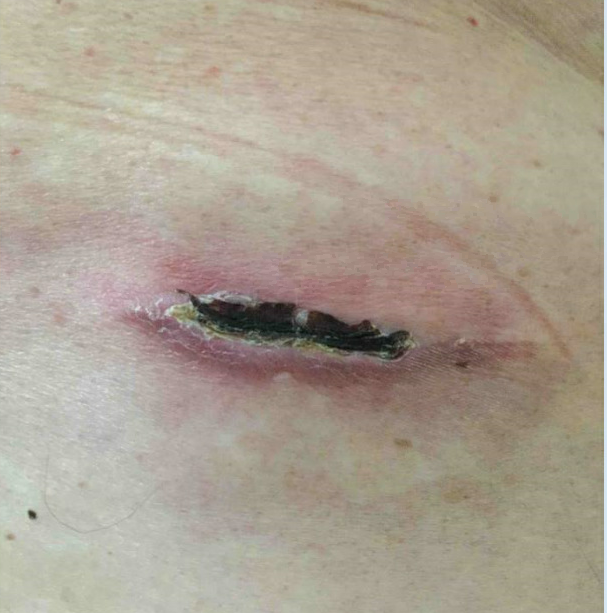
Figure 1. Post-operative examination of implanted pulse generator.
a. Superficial surgical site infection (SSI)
b. Deep, uncomplicated SSI
c. Deep, complicated SSI
a. Complete blood count (CBC)
b. Magnetic resonance imaging (MRI)c. Ultrasoundd. Blood cultures
3. What factor(s) may predispose her to a possible surgical site infection?
Longer surgical time and trial duration greater than 5 days have been shown to be risk factors for infection, and she has an extended trial period of 10 days. While not demonstrated as risk factors for SCS infection in the limited studies to date, commonly associated variables such as uncontrolled diabetes mellitus (DM) and smoking should raise caution for surgical site infection. Well controlled DM may also pose a small increased risk of infection. In practice, many use a HgbA1c cutoff of 8 before proceeding with implantation.
4. Given reassuring labs, you decide to move forward with oral antibiotic therapy. Which treatment regimen do you choose?
A first-generation cephalosporin for 7-10 days is the antibiotic of choice for a superficial infection. This choice affords appropriate gram-positive coverage. Staphylococcus, streptococcus, and enterococcus species are most common. Methicillin-resistant staphylococcus aureus (MRSA) coverage can be considered based on colonization.
5. In SCS associated infections, when is explant indicated?
Explant is indicated for any deep infection.
Redness and swelling of the surgical site initially improve with a 7-day course of oral antibiotics. However, one week later, she again has worsening redness, continued back pain along with a small amount of purulent drainage. During neurological assessment, manual muscle testing reveals 4/5 strength in the right hip flexors and quadriceps muscles with a diminished right patellar reflex. Heart rate is 85, blood pressure is 130/83, respiratory rate is 14, and temperature is 37.3.
6. Besides repeat serum labs, what other laboratory testing do you request?
Epidural abscess vs deep infection is now at the top of your differential. Deep infection is suspected if dehiscence, purulent drainage, or erosion of device components is present. Epidural abscess is considered when there is ongoing/worsening back pain with neurological deficits. Common presenting clinical features of epidural abscess include back pain (66.8%), motor weakness (52.0%), fever (43.7%), sensory abnormalities (40.0%), and bowel/bladder incontinence (27.1%). The most common abscess location is the lumbar spine (48%). If epidural abscess is initially suspected, pre-explantation imaging is warranted. If only deep infection is initially suspected, pre-explantation imaging is not necessary, but it is good practice to obtain post-explantation advanced imaging to evaluate for complication such as epidural abscess or osteomyelitis.
7. What imaging study would be indicated for further investigation of epidural abscess?
8. Imaging shows an epidural abscess at the L3-L4 level. What are your next steps in management?
9. The patient is seen in the pre-operative area immediately prior to planned laminectomy with incision and drainage of a known abscess. They are endorsing moderate-to-severe pain and diminishing effects of a short course of opioids they were prescribed in the setting of this complication. You discuss their care with their anesthesiologist who decides this patient will benefit from a multimodal perioperative pain management plan. Which of the following medications would be helpful adjuncts to consider?
All three options (a, b, and c) are reasonable to consider. Most of these medications have been studied in the setting of lumbosacral fusion and studies have both supported and refuted their use. Importantly, there is no research that studies our patient’s clinical situation. A recent RCT in patients chronically on opioids by Nielsen et al. revealed ketamine reduced daily oral morphine equivalents in the one-year period after fusion. Murphy et al. found reduced hydromorphone use on postoperative day 1-3 in patients randomized to intraoperative administration of methadone versus standard hydromorphone. De Winter et al. randomized patients to intraoperative lidocaine or placebo and found no difference in postoperative opioid consumptions, however, lidocaine is likely safe and could still be an additional adjunct in the appropriate context.
10. If instead, there was no epidural abscess and the patient had a normal neurological examination (deep infection), after explanation of the SCS device, what antibiotic regimen do you pursue?
Empiric coverage is generally necessary for gram positive species only. This may be directed by local antibiogram. Treatment should then be narrowed based on serum and intra-operative culture data. Consultation with an infectious disease specialist may be warranted with any complicated infection. After explantation, 7-10 days of therapy indicated (longer in the case of a complicated infection). In a clinically stable patient with uncomplicated deep infection, initiation of antibiotics may be deferred until after explanation and collection of operative cultures.
11. What are preventative measures to reduce risk of infection?
12. Your patient returns to the clinic after completion of her antibiotic course and resolution of her deep infection. Her pain has markedly increased since explantation and she asks if she can have another spinal cord stimulator implanted. How do you counsel her?
Bibliography
Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and Surgical Management of Spinal Epidural Abscess: A Systematic Review. Neurosurg Focus. 2014;37(2):E4.
Babic M, Simpfendorfer CS, Berbari EF. Update on Spinal Epidural Abscess. Curr Opin Infect Dis. 2019;32(3):265-271.
Bendel MA, O'Brien T, Hoelzer BC, et al. Spinal Cord Stimulator Related Infections: Findings From a Multicenter Retrospective Analysis of 2737 Implants. Neuromodulation. 2017;20(6):553-557.
Deer TR, Provenzano DA, Hanes M, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Recommendations for Infection Prevention and Management [published correction appears in Neuromodulation. 2017 Jul;20(5):516]. Neuromodulation. 2017;20(1):31-50.
Deer TR, Provenzano DA. Recommendations for Reducing Infection in the Practice of Implanting Spinal Cord Stimulation and Intrathecal Drug Delivery Devices: A Physician's Playbook. Pain Physician. 2013;16(3):E125-E128.
Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, Van de Velde M, Rex S. Systemic Lidocaine Fails to Improve Postoperative Morphine Consumption, Postoperative Recovery and Quality of Life in Patients Undergoing Posterior Spinal Arthrodesis. A Double-Blind, Randomized, Placebo-Controlled Trial. Br J Anaesth. 2017;118(4):576-585.
Esquer Garrigos Z, Farid S, Bendel MA, Sohail MR. Spinal Cord Stimulator Infection: Approach to Diagnosis, Management, and Prevention. Clin Infect Dis. 2020;70(12):2727-2735.
Hoelzer BC, Bendel MA, Deer TR, et al. Spinal Cord Stimulator Implant Infection Rates and Risk Factors: A Multicenter Retrospective Study. Neuromodulation. 2017;20(6):558-562.
Murphy GS, Szokol JW, Avram MJ, et al. Clinical Effectiveness and Safety of Intraoperative Methadone in Patients Undergoing Posterior Spinal Fusion Surgery: A Randomized, Double-blinded, Controlled Trial. Anesthesiology. 2017;126(5):822-833.
Nielsen RV, Fomsgaard JS, Nikolajsen L, Dahl JB, Mathiesen O. Intraoperative S-Ketamine for the Reduction of Opioid Consumption and Pain One Year After Spine Surgery: A Randomized Clinical Trial of Opioid-Dependent Patients. Eur J Pain. 2019;23(3):455-460.
Wetherington B, Khan TW. Epidural Abscess During a Spinal Cord Stimulator Trial: A Case Report. Pain Pract. 2019;19(1):57-60.
Leave a commentOrder by
Newest on top Oldest on top