Approach to a Patient with Cancer Pain: A Problem-Based Learning Discussion
1. Given this patient's medical history and review of systems, what is the most worrisome etiology of his back pain? What are other differentials?
The top of the differential should include prostate cancer recurrence with metastasis to the bones of the lumbar spine. Given his lack of known trauma or injury, in addition to the symptoms of unintentional weight loss, night sweats, and new onset of constant progressive low back pain in the setting of previously treated prostate cancer, bone metastasis to the lumbar or sacral spine is highly likely.1 Among patients with recurrent or advanced cancer, 60%-84% experience some degree of bone pain (approximately 450,000 Americans annually).2 After the lungs and liver, bone is the third most likely location of cancer metastasis for all cancers, of which multiple myeloma, breast, prostate, lungs, thyroid, kidney, and ovarian cancers make up the majority of cases.2 As many as 65% of all bony metastases come from breast and prostate cancers.2 Bony metastasis to the vertebral bodies is the most common location, followed by the pelvis, long bones, and cranium.2 Location and number of bony lesions do not correlate clinically with severity of pain. Some patients with disseminated disease have low levels of pain while others with one tumor can report severe, debilitating pain. Therefore, an individualized approach is warranted, with a strong history, physical exam, and treatment plan.2
Other differentials to consider, which may be less likely given the patient’s history, are vertebral compression fracture, lumbar spondylosis, spinal stenosis, disc herniation, and vertebral osteomyelitis. These aspects of the differential diagnosis are important. Although bony metastasis is highly likely, diagnostic bias can lead the physician to miss other, potentially key points of information from the history or physical exam. Covering the pathophysiology and mechanism of these other diseases is beyond the scope of this problem-based learning discussion (PBLD) but should be considered in real life. The physician must elicit key elements of the history, which continue to make other diagnoses more or less likely, and therefore home in on the correct diagnosis.
2. What are “red flag symptoms,” and does this patient have any. If so, what are they? How does the presence of red flag symptoms change your approach to management?
Red flag symptoms include unexpected weight loss, recurrent fevers and chills, unrelenting and severe pain, bowel or bladder incontinence, saddle anesthesia, progressive neurological deficit, and pain that is worst at night.
This patient does have the red flag symptoms of unintentional weight loss, night sweats, and new onset of constant progressive low back pain that wakes him up at night. In the setting of previously treated prostate cancer, bone metastasis to the lumbar or sacral spine is highly likely.
When red flag symptoms are present, diagnostic evaluation must be more aggressive and diligent. An in-depth history and physical exam are important to understanding risk factors (ie, personal or family history of cancer, IV drug abuse, tuberculosis exposure, or prolonged steroid use). Screening labs, such as a comprehensive metabolic panel and complete blood count, should be obtained, in addition to imaging. The case should be discussed with the patient’s PCP to ensure no loss to follow up and that other screening labs, imaging, and procedures are obtained.
3. Given the patient's red flag symptoms, the patient’s PCP is concerned about prostate cancer recurrence. How is cancer pain different from other types of chronic pain? How is bone pain described?
Cancer pain can be categorized as acute versus chronic, somatic versus neuropathic, and disease- versus treatment-related. Most cancer pain is continuous over time, with some variation in intensity, particularly at night.2 Without intervention, it rarely disappears completely and often intensifies with time.2 Cancer pain is also frequently associated with intermittent paroxysms of pain that occur with activity, and it can be associated with episodic breakthrough pain that is not always manifested by a specific trigger. Patients are often otherwise stable on their baseline pain regimens with good control.2 In these scenarios, they will likely need supplementation of their baseline regimens with higher levels of therapy or care. Nearly 75% of patients with bony metastasis will have breakthrough pain.2
Bone pain is described as a deep boring quality that can ache or burn. The pain is accompanied by episodes of stabbing discomfort. Pain is usually worst at night and with movement. Metastasis or primary cancer into the highly innervated periosteum may give bone pain neurogenic qualities, which add to the complexity and severity of the pain and management.3 In patients with metastatic bone disease, there is always concern of spinal instability, which can cause severe pain of a mechanical origin. Patients with spinal instability are only comfortable when lying absolutely still. In this scenario, radiotherapy or systemic treatment will not relieve the pain; stabilization may be required for pain relief.3
4. The patient’s PCP is concerned about metastasis to the patient’s spine or pelvis. What is the most sensitive and specific imaging to detect bone metastasis?
Magnetic resonance imaging (MRI) is reported to be 91% sensitive and 95% specific for detecting bone metastasis.4 MRI reveals bone metastases in the bone marrow spaces before any changes in internal bone structure can be detected by computerized tomography (CT). T1-weighted and STIR sequences alleviate the need for IV contrast, allowing patients with poor renal function to undergo MRI. MRI also does not involve ionizing radiation. MRI can detect metastasis in the extraosseous soft tissue such as metastasis compressing the spinal cord.4
Positron emission tomography combined with CT (PET-CT) is a nuclear medicine technique that has been shown to have 90% sensitivity and 97% specificity in detecting bone metastasis.4 A radioactive tracer is injected intravenously prior to imaging so that the tracer can reach locations where metabolism is occurring. After a certain amount of time, PET-CT is performed. Imaging illustrates tumor metabolism all over the body, including the bone. The visualization of glucose metabolism by positron-emission tomography with 18F-fluorodeoxyglucose, coupled with a simultaneously obtained CT (18F-FDG-PET-CT), is now a standard diagnostic technique in oncology. The 18F-FDG-PET-CT can be used for complete staging.4
5. The patient's PCP orders an MRI of the patient's thoracic spine, lumbar spine, and pelvis and calls his oncologist to let them know they are worried about prostate cancer recurrence with metastasis. The patient notes he will be unable to tolerate the pain while waiting to schedule and get scan results. He states he is taking ibuprofen and acetaminophen 3-4 times a day, yet he is experiencing excruciating pain. What is the World Health Organization (WHO) Stepladder approach to manage cancer pain? What should be the PCP’s next step in managing the patient’s pain based on the WHO stepladder?
The WHO Stepladder approach used to be a three-step process to manage cancer pain, but recently it has been updated. It is now a four-step process as seen in figure below.
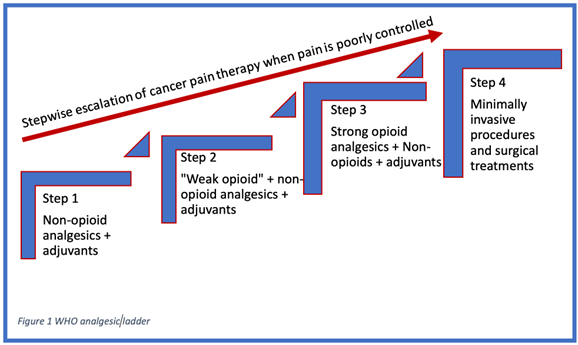
Figure 1. Visual representation of the WHO analgesic stepladder1,5
Step 1 is to manage the pain with non-opioid medication, with or without adjuvants.5 This step is for patients with complaints of pain that is 1-3 on a 10-point scale. Examples of these medications are APAP and non-steroidal anti-inflammatories (NSAIDS) such as ibuprofen or naproxen. Dosage of these medications can be increased to recommended daily maximums, and they obey first order pharmacokinetics.1
Step 2 is to add weak opioids to non-opioids and adjuvant if pain persists.5 This class of medications often includes “weak” opioids and opioid-combination medications such as APAP/codeine, APAP/hydrocodone, APAP/oxycodone, ibuprofen/hydrocodone, and tramadol.1 Of those listed, only tramadol is a truly weak opioid agonist; the other medications listed have similar potency to morphine but are considered weak due to the maximum dose constraints from their APAP or NSAID components.1 The combination opioid medications from Step 2 also obey first order kinetics and may be dosed up to the daily recommended maximums.1 Of note, there is no evidence to date that the maximum dose of any Step 2 combination drug is more efficacious than any other. If a patient's pain is poorly controlled on the maximum dosing of a Step 2 medication, escalation to Step 3 should not be delayed as it will only prolong time until pain control is achieved and place the patient at risk for overdosing on step 2 combination medications (> 4 g of APAP per day or 2,400 mg of ibuprofen).1
Step 3 is to replace weak opioids with strong opioids. This step combines strong opioids with or without adjuvants to achieve pain control.5 Step 3 is composed of the pure opioid agonists such as morphine, hydromorphone, methadone, fentanyl, and oxycodone. All available pure opioid agonists’ analgesic potency can be converted into oral morphine equivalents (OMEs) to determine conversions between pure opioid agonists.1 The same adjuvants from Step 1 are included in the WHO Step 3 level of pain control. In contrast to Step 1 and 2 medications, there is no upper limit or ceiling effect when titrating medications for pain control in Step 3. However, the physician needs to be aware of adverse effects and signs of abuse, dependance, and inadequate analgesia and manage accordingly.1
Step 4 has been added for patients with significant pain. Noninvasive routes of drug delivery are maintained as long as possible, but interventional procedures, including blocks, neuromodulation, drug delivery systems, and/or neurolysis should be used for patients whose pain is not controlled or those having significant side effects from medications.5 Several studies have shown that up to 90% of individuals will get adequate analgesia for their cancer-related pain from the traditional WHO three step ladder of analgesia in cancer pain.1 Step 4 was created for the remaining patients who have significant medication side effects or intractable, uncontrolled pain despite maximal pure oral opioid agonist therapy + adjuvants. Step 4 starts with subcutaneous or IV administration of opioid analgesics with the goal of more rapid titration of serum concentration to determine analgesic needs, avoidance of first pass metabolism compared to oral analgesics, or avoidance of unwanted oral side effects of high doses of opioid.1 In select patients, more invasive techniques may be needed to manage cancer pain. Blocks, continuous intrathecal opioid administration via an implanted intrathecal pump, neuroablative techniques, neuromodulation, and spinal bone stabilization and augmentation are among the tools used in Step 4.
The PCP should start the patient on weak opioids with adjuncts to help manage the pain.
6. The patient's PCP orders the patient Percocet 5, 325 mg, every 6 hours and instructs the patient to continue taking ibuprofen and acetaminophen while making sure that he doesn’t exceed the maximum dosing. The patient calls after a week and states that the medication he has been taking has helped some but his pain at night is still really bad and keeps him from sleeping. The patient also states that he is in excruciating pain when he walks more than 15 minutes. As a result, he has been avoiding things he normally likes to do. The patient's PCP decides to escalate his opioid therapy to oral oxycodone in addition to the ibuprofen and acetaminophen. What are the side effects of opioid therapy, and how would you treat them?
Opioids are one of the main tools of treatment for cancer pain, but they come with many side effects.
GI: An estimated 40%-45% of patients on opiate therapy experience constipation. Constipation is due to the opioid’s stimulation of kappa and mu receptors in the GI tract. Constipation can be so severe that it can lead to bowel obstruction. An estimated 25% of people taking opioids develop nausea, and some patients can experience vomiting, abdominal cramping, and bloating. The overall risk of GI bleed with opioids is the same as that of NSAIDs.6
Nausea and vomiting can be managed with antiemetics. Constipation can be treated with a regular bowel regimen of laxatives and stool softeners. For constipation that is resistant to more conventional bowel regimens, a peripherally acting Mu-receptor antagonist, such as methylnaltrexone, may be appropriate.
Respiratory: Opioid therapy can lead to respiratory depression. The respiratory effects of opioids are dose dependent. An estimated 10% of patients on chronic opioid therapy experience some degree of hypoxemia.6
Cardiovascular: Hypotension and bradycardia, along with respiratory depression, can be seen with opioid overdoses.6
Central nervous system: Opioids can cause dizziness and sedation, which can lead to falls and injury. Hyperalgesia can be seen in patients taking chronic opioid therapy.6
Sedation, respiratory depression, and opioid toxicity can be treated with specific mu-receptor antagonists (naloxone) and supplemental oxygen. Intravenous bolus of naloxone dose of 1-4ug/kg can promptly reverse sedation, respiratory depression, and analgesia.
Endocrine system: Patients using chronic opioids have hyperfunctioning hypothalamic pituitary adrenal axis and hypofunctioning hypothalamic pituitary gonadal axis. Patients using chronic opioids have decreased gonadotropin-releasing hormone. In males, this can lead to sexual dysfunction, infertility, fatigue, and decreased testosterone. In females, this can lead to decreased levels of estrogen, low follicle stimulating hormone, and increased prolactin, which causes osteoporosis, oligomenorrhea, and galactorrhea.6
7. Results of the patient's MRI reveal evidence of metastasis to the patient’s L2, L3, and L4 vertebral bodies with no compression fractures or compression of any nerve roots of the lumbosacral plexuses. What interventional techniques are used in the management of cancer pain? What techniques are more frequently used in patients with spinal metastasis?
Several interventional procedures can be used to manage cancer pain. These include nerve blocks, continuous intrathecal opioid administration via an implanted intrathecal pump, neuroablative techniques, neuromodulation, and spinal bone stabilization and augmentation. Interventional techniques can significantly reduce pain levels, leading to decreased analgesic requirements. Typically, conservative management has been exhausted at this time, as demonstrated by the WHO stepladder approach.5 When assessing a patient's candidacy for an interventional procedure, keep in mind their primary tumor, site(s) of lesion(s), social situation (specifically for an intrathecal system), neurological function, and prognosis.
Nerve blocks: Depending on the location of the pain, peripheral or neuraxial blocks can be used. Peripheral nerve blocks are performed at anatomic sites away from the central neuraxis. Thoracic pain can be managed with intercostal or paravertebral nerve blocks. Abdominal and pelvic pain can be managed with celiac plexus, splanchnic nerve block, superior hypogastric block, and ganglion impar blocks respectively. Diagnostic blocks can initially be performed with local anesthetics to verify the blocks’ therapeutic effects. If beneficial, the outcome of blocks can be extended with chemical neurolysis, cryoablation, and radiofrequency ablation.7
Chemical neurolysis: Chemical neurolysis with alcohol or phenol can be used to perform neuraxial neurolysis of celiac plexus, superior hypogastric, and or ganglion impar. Chemical neurolysis is irreversible, but pain can return in 6-9 months. Neurolysis procedures can cause postural hypotension and bowel and bladder incontinence.7
Intrathecal drug delivery: A much lower concentration of opioid analgesic can be administered in the intrathecal space to allow for equal or better pain control while minimizing medication side effects. Of the common epidural or intrathecal opioids administered, a good estimate of potency is that 10 mg of IV morphine is equivalent to approximately 1 mg of epidural morphine and 0.1 mg of intrathecal morphine. Administering opioids in epidural or intrathecal space enables the opioid to reach its receptor (primarily mu) in the central nervous system (spinal root and periaqueductal gray in the brain) directly, diminishing the side effects from enteral opioids, which also have a strong effect on peripheral opioid receptors in the gut and other abdominal organs. An intrathecal drug delivery system is implanted under sedation/general anesthesia. A catheter is placed into the intrathecal space and then tunneled percutaneously to connect to an implanted intrathecal delivery device. The device contains the medication, which can be refilled percutaneously when the drug reservoir runs low. Risks of intrathecal drug delivery include spinal infection, hematoma, neurologic injury, cerebrospinal fluid leak, and development of catheter granuloma or obstruction.7
Spinal cord stimulator (SCS): The purpose of an SCS is to modulate nerve activity through targeted delivery of an electrical stimulation. SCS is commonly used for nonmalignant pain conditions like failed back surgical syndrome, limb ischemia, and complex regional pain syndrome, but multiple reports have described its successful use in cancer patients. Specifically, SCS has been shown to provide pain relief for individuals suffering from axial/appendage bone pain related to metastasis, neuropathies due to tumor invasion or compression, and treatment-related pain.8 SCS implantation involves placement of electrodes in the epidural space overlying the dorsal surface of the spinal cord. The electrodes are then connected to an impulse generator that is implanted subcutaneously. Prior to implantation, SCS can be trialed to assess its therapeutic effect. Risks of SCS include device malfunction, infection, lead migration, and cerebrospinal fluid leak during implantation.7
Vertebral augmentation (vertebroplasty or kyphoplasty): Bone metastasis can cause vertebral compression fracture. If metastasis does not involve the spinal canal, vertebroplasty or kyphoplasty can be performed to alleviate back pain caused by vertebral compression fracture.9 This percutaneous spinal procedure involves injecting bone cement (polymethylmethacrylate) through a small hole in the skin into a fractured vertebrae to stabilize the fragmented bone. Kyphoplasty uses a balloon to expand the vertebrae and create a void to administer the cement under low pressure. The exact mechanism of analgesia is not fully understood, but it is believed that pain relief is either due to the mechanical support the cement provides and or exothermic reaction of the cement itself.10
Radiofrequency ablation (RFA) and vertebroplasty: Studies have demonstrated that RFA and vertebroplasty in combination are significantly more effective in reducing pain associated with axial metastasis than vertebroplasty alone. RFA uses high-frequency, alternating current that passes from the electrode needle to adjacent tissues, causing friction heating. The heat can cause tissue necrosis, including necrosis of metastatic tumor cells and adjacent sensory nerve fibers. RFA can arrest bone damage and inhibit pain-inducing osteoclastic activity, and it also promotes the release of different cytokines and biochemical factors. RFA provides curative treatment for benign and malignant lesions measuring up to 3 cm. Vertebroplasty is performed after RFA, depending on lesion size and location, as the stability of the vertebral column can be compromised.11
Radiotherapy, chemotherapy, isotopic therapy, bisphosphonate therapy, pharmacotherapy, RFA, and palliative surgery have been used to treat spinal metastases.11 The mode of treatment is individualized to the patient, comorbidity, amount of tumor burden, and prognosis. If the patient only has bone metastasis to one or two levels of the spine, then radiation therapy and RFA plus vertebroplasty would be a good treatment option. If the patient has multiple lesions and pain generators in other areas, an intrathecal pain pump might be a better treatment option as RFA plus vertebroplasty would not address other causes of pain.
8. The patient sees his oncologist with his MRI results and asks if radiation therapy will help with his back pain. What is the role of radiation therapy in cancer pain? What are its side effects?
Radiation therapy is a physical agent (ionizing radiation) used to destroy cancer cells. High-energy radiation damages genetic material preventing it from replicating. Radiation therapy can be curative or palliative to relieve pain and/or symptoms from metastasis. It can be complementary to chemotherapy, surgery, and analgesic drug therapies to optimize pain control. It is most commonly used for bone metastasis. The radiation dose should be administered in the fewest fractions possible to promote pain control during and after treatment. The goal of radiation therapy is to maximize the radiation dose to the abnormal cells.12
Radiation therapy does not have complete specificity. It damages both normal and cancer cells; therefore, the treatment may exacerbate or even cause new pain. Reactions to radiation therapy often start during the second to third week of treatment. Reactions may be long term or last for several weeks after the final treatment. Side effects are usually localized to the area of treatment. Common physical side effects of radiation therapy include skin changes and fatigue. Skin changes range from dryness, itchiness, blistering, and peeling of skin to more serious problems. Skin changes usually go away after a few weeks of treatment.12
9. The patient is very anxious and depressed because of the MRI and reoccurrence of cancer. What are the psychosocial factors involved in the treatment of cancer pain? What role do they play in an overall treatment plan?
Psychosocial factors are a large part of the suffering that patients with chronic and cancer pain experience. Suffering is a large component of chronic pain that must be addressed in a complete pain plan.
Concept of “total pain”: This is the idea that the relationship between physical manifestations, mental distress, and social and emotional difficulties associated with pain are essential in understanding the total pain and suffering a patient with chronic pain experiences. A concept introduced into the world in the 1960s with the advent of palliative care and hospice medicine has been expanded on and adapted to the world of chronic pain medicine to encompass a multidisciplinary approach to the whole patient's experience of pain and suffering.13
Anxiety and depression: Anxiety and depression are a large part of pain and suffering that can be treated traditionally with medications and therapy. In the context of a multimodal pain treatment plan, evidence from randomized control trials demonstrates the therapeutic effects of mind-body practices for the treatment of suffering caused by anxiety and depression in relation to chronic and cancer-related pain. Mind-body practices consist of mediation (mindfulness-based stress reduction), yoga, tai chi, guided imagery, biofeedback, and music therapy.14
Family: The role of family in the mental and physical support and treatment of a patient with chronic cancer pain cannot be understated. For patients with cancer, family caregiving is a large part of healthcare delivery, especially pain medication management in the outpatient setting.15 Involving and educating family caregivers will continue to be a growing component of a strong multidisciplinary plan for managing cancer-related pain.
Spirituality: Patients’ individual belief systems should be taken into consideration when developing a pain treatment plan. Individuals with strong belief systems, unique cultural backgrounds, and strong spiritual needs will likely benefit from integrative therapies such as those stated above.14 Integration of core components of patients’ belief systems (eg, pastor or priest, familial presence, distinguished members of a patient's community) are shown to have a significant impact on the suffering aspect of chronic and cancer pain.
Existential: Pain is very anxiety-provoking from a societal level. It invokes feelings of fear, loss of autonomy, death, and decay, and it is greatly feared by the public.16 Many cancer patients who experience chronic pain correlate pain intensity with fear of dying and fear of the future. Patients often express that the fear of pain progression is worse than the knowledge of impending death and the cancer diagnosis itself.15 In many cultures and religions around the world, pain is portrayed as a trial, punishment, or warning for living an unjust or immoral life, further leading to distress, particularly with those who lack a support system.16 Physicians need to be aware of these variabilities in the spiritual and cultural aspects of pain. These vulnerable patients need reassurance and resources.References
- Benzon H, Raja SN, Liu SS, et al. Essentials of Pain Medicine. Elsevier: 2017.
- Zajączkowska R, Kocot-Kępska M, Leppert W, et al. Bone pain in cancer patients: mechanisms and current treatment. Int J Mol Sci 2019;20(23):6047. https://doi.org/10.3390/ijms20236047
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer treatment reviews 2001;27(3)165-76. https://doi.org/10.1053/ctrv.2000.0210
- Heindel W, Gübitz R, Vieth V, et al. The diagnostic imaging of bone metastases. Dtsch Arztebl Int 2014;111(44):741-7. https://doi.org/10.3238/arztebl.2014.0741
- Anekar AA, Cascella M. WHO analgesic ladder. [Updated 2022 May 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Retrieved on October 18, 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554435/
- Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy: a practitioner's guide. Prim Care Companion CNS Disord 2012;14(3). https://doi.org/10.4088/PCC.11m01326
- Yalamuru B, Weisbein J, Pearson ACS, Minimally-invasive pain management techniques in palliative care. Ann Palliat Med 2022;11(2):947-57. https://doi.org/10.21037/apm-20-2386
- Hagedorn JM, Pittelkow TP, Hunt CL, et al. Current perspectives on spinal cord stimulation for the treatment of cancer pain. J Pain Res 2020;13:3295-305. https://doi.org/10.214 7/JPR.S263857
- Denaro V, Longo UG, Maffulli N, et al. Vertebroplasty and kyphoplasty. Clin Cases Min Bone Metab 2009;6(2):125-30.
- Siemionow K, Lieberman IH. Vertebral augmentation in osteoporosis and bone metastasis. Curr Opin Support Palliat Care 2007;1:323–327.
- Yildizhan S, Boyaci MG, Rakip U, et al. Role of radiofrequency ablation and cement injection for pain control in patients with spinal metastasis. BMC Musculoskel Disord 2021;22(1), 912. https://doi.org/10.1186/s12891-021-04799-0
- Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9(3):193-9. https://doi.org/10.7150/ijms.3635
- Saunders C. Care of patients suffering from terminal illness at St. Joseph’s Hospice. Nursing Mirror 1964;14:7-10.
- Deng G. Integrative medicine therapies for pain management in cancer patients. Cancer J 2019;25(5):343-8. https://doi.org/10.1097/PPO.0000000000000399
- Ferrell BR. Family caregiving and cancer pain management. Anesth Analg 2019;129(5):1408-13. https://doi.org/10.1213/ANE.0000000000003937
- Strang P. Cancer pain: a provoker of emotional, social and existential distress. Acta Oncol 1998;37(7-8):641-4. https://doi.org/10.1080/028418698429973
Leave a commentOrder by
Newest on top Oldest on top