Facet Joint Pain
Authors
Srinivas Chiravuri, MD
Director, Pain Medicine Fellowship
Director, Neuromodulation
Assistant Professor
University of Michigan Health System
Ann Arbor, MI
Chad M. Brummett, MD
Director, Adult Pain Research
Assistant Professor
Department of Anesthesiology
University of Michigan Health System
Ann Arbor, MI
Lynn Kohan, MD
Pain Management Fellowship Director Department of Anesthesiology
University of Virginia Health System
Charlottesville, VA
Introduction
Spine pain is a common problem throughout the world and is a significant cause of pain and loss of function. Lifetime prevalence estimates are as high as 84% for back pain[1] and 67% for neck pain[2]. Low back pain (LBP) is a leading cause of disability, with lost wages estimated at over $200 billion in 2002–2004[3]. The etiology is usually multifactorial with involvement of muscles, ligaments, discs, nerve roots, and zygapophysial (facet) joints. The facet joint is a potential source of headaches and pain in the neck, shoulder, mid back, low back, and leg. The economic burden of low back and neck pain is also substantial with an estimated cost of $86 billion in health care expenditures in the United States.[51] Facet injections were found to be the 2nd most common procedure performed in pain clinics in the United States.[62] Spine injections, particularly facet joint interventions, are one of the major contributors to the explosive growth and increasing expenditures for patients with chronic pain. Therefore facet joint interventions have become a focus for payers, public policy health experts, and researchers.[52] Manchikanti et al found that facet joint interventions increased by 308% per 100,000 Medicare beneficiaries from 2000 to 2010.[53]
Anatomy
Facet joints are true synovial joints formed from the superior articular process of one vertebra and the inferior articular process of the vertebra above. The volume capacity of the joints is 1–1.5 mL and 0.5–1.0 mL in the lumbar and cervical regions, respectively.[5] The medial branch is a branch of the posterior division of the spinal nerve root and provides innervation of the facet joints and paraspinous muscles (Figure 1).
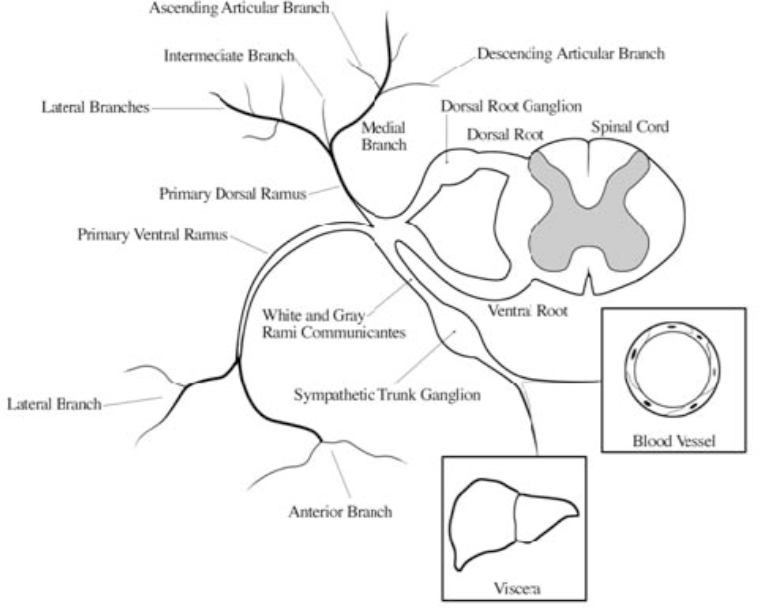
Figure 1. Nerve root division. The smaller posterior division of the nerve root divides into lateral, intermediate, and medial branches. The medial branch is the largest of the three and provides innervation to the facet joint and paraspinal muscles.
From Cohen SP, Raja SN: Pathogenesis, diagnosis and treatment of lumbar zygapophysial [facet] joint pain. Anesthesiology 2007;106:591-614.
The facet joint is innervated by both the medial branch from the level above and the medial branch from the same level (i.e. the L3-4 facet joint is innervated by the medial branches from the L2 and L3 nerve roots; Figure 2).
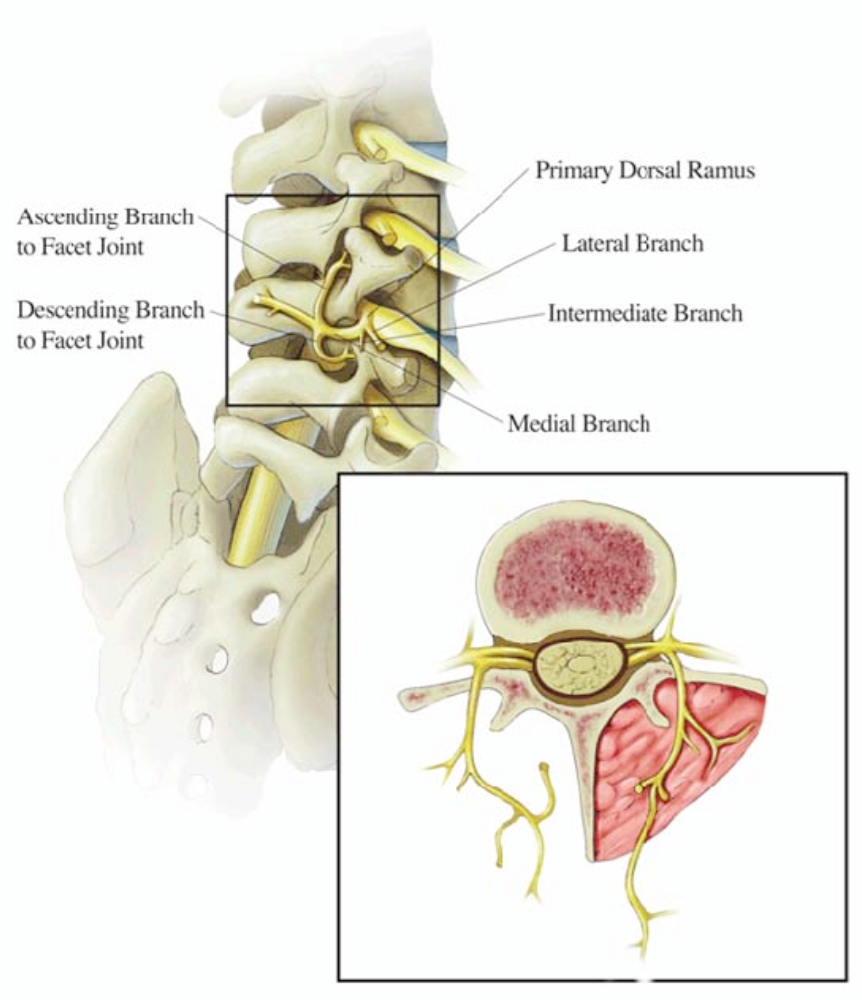
Figure 2. Lumbar facet joint innervation. The facet joint is innervated by the medial branch from the nerve root at the same level and the level above.
From Cohen SP, Raja SN: Pathogenesis, diagnosis and treatment of lumbar zygapophysial [facet] joint pain. Anesthesiology 2007;106:591-614
Lumbar Spine
The medial branch is reasonably consistent in its location in the lumbar spine. The medial branch lies at the junction of the transverse and superior articular processes of the vertebral level below (i.e. the L4 medial branch sits on the bone of the L5 vertebral body). The nerve traverses the dorsal leaf of the intertransverse ligament of the transverse process and courses underneath the mamilloaccessory ligament, splitting into multiple branches as it crosses the vertebral lamina (Figure 2). The mamilloaccessory ligament can become calcified and be a source of nerve entrapment, especially at L5. The main variation in the lumbar spine is the L5 level, where it is the primary dorsal ramus itself that is amenable to blockade on the sacral ala.[6]
Thoracic Spine
Thoracic medial branches assume different courses depending on the level.[7] The superior- lateral corner of the transverse process is the most consistent point for blockade in the thoracic region (Figure 3 a–b). Stimulation of the multifidus muscle cannot be used to confirm needle position prior to denervation in the thoracic region, as the nerve circumvents the muscle.
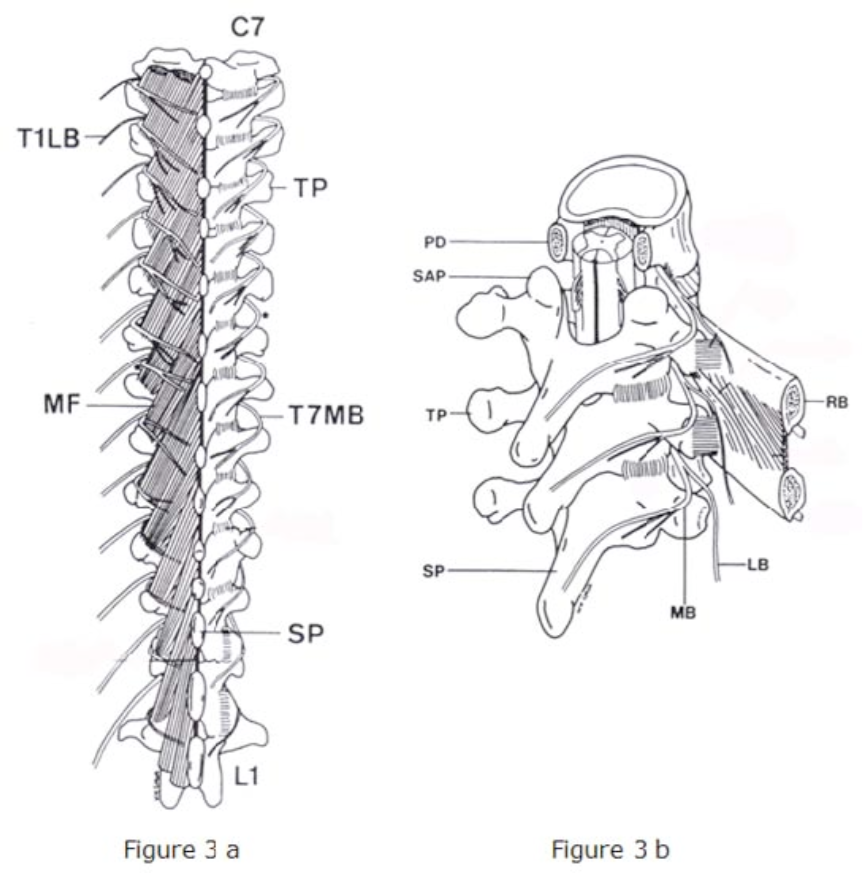
Figure 3 a–b. Thoracic facet joints. The medial branch in the thoracic region wraps laterally, thereby making the superiorlateral corner of the transverse process as the optimal location for blockade. Figure 3a demonstrates a posterior view, while 3b shows the thoracic medial branches from an oblique view. LB = lateral branch; MB = medial branch; TP = transverse process; SAP = superior articular process; SP = spinous process.
From Chua WH, Bogduk N: The surgical anatomy of thoracic facet denervation. Acta Neurochirugia 1995;136:140–144
In addition, the medial branch nerves in the thoracic region are somewhat variable and may not always be in contact with bone as seen in Figure 3c.
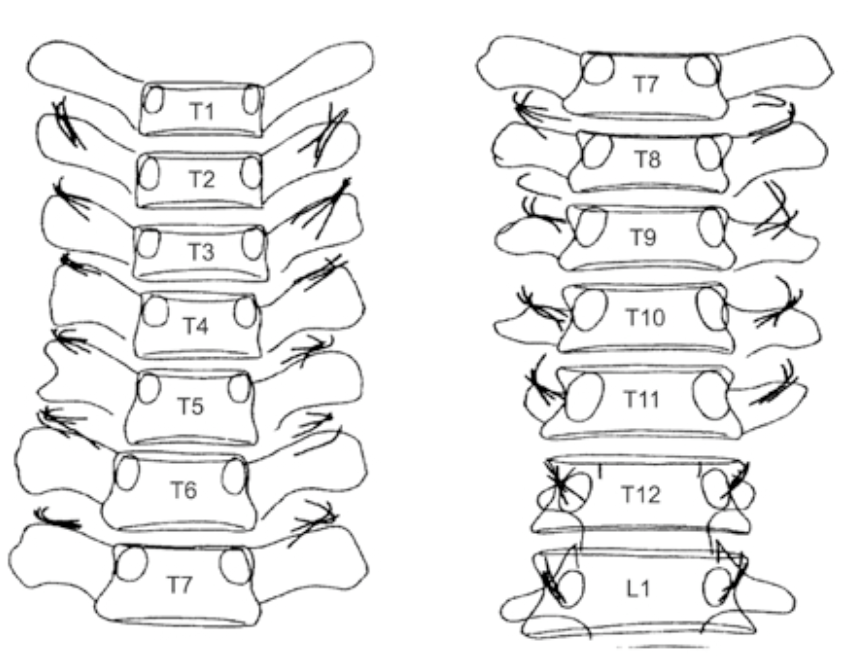
Figure 3c. Thoracic medial branches. Illustration of the varying positions of the medial branches in the thoracic spine.
From Bogduk N ( 2nd ed). Practice Guidelines for Spinal Diagnostics and Treatment Procedures,. International Spine Injection Society (ISIS) 2004.
Cervical Spine
Similar to the lumbar and thoracic regions, the C3-4 through the C7-T1 joints receives innervation from the medial branches at the same level and the level above. The nerves curve around the waist of the articular pillars (Figure 4), except at C7 and C8 where the anatomy is more variable.[8] The majority of the innervation of the C2-3 joint comes from the dorsal ramus of C3. The C3 dorsal ramus divides into two separate medial branches, the larger of which is known as the third occipital nerve. The C2 dorsal ramus divides into up to five branches, the largest of which is the greater occipital nerve. Pathology involving branches of the C2 and C3 dorsal rami are a common source of occipital headaches.
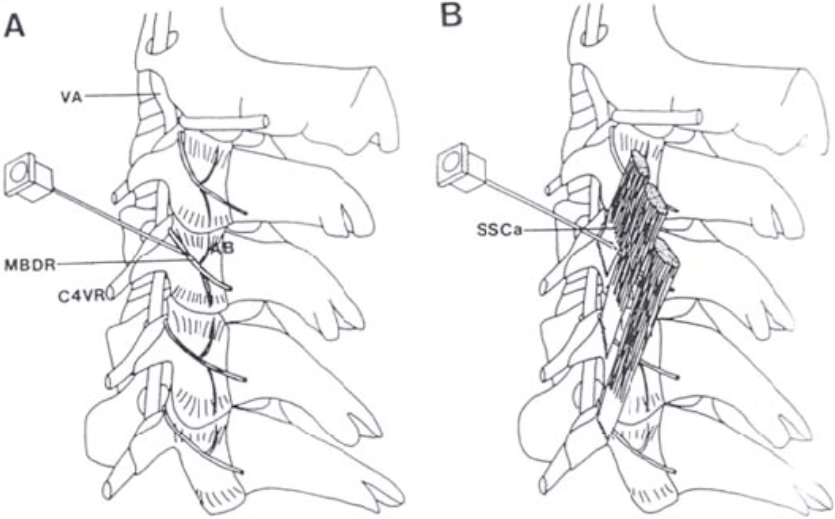
Figure 4. Cervical facet joints. The anatomy and innervation of the cervical facet joints is more complicated. This sketch depicts a lateral approach for blockade of the cervical medial branch. At most levels, the medial branch in the cervical region wraps around the waist of the articular pillars before dividing into articular branches (AB) to innervate two facet joints. It is held tightly in place by the semispinalis capitis (SSCa). C4VR = 4th cervical ventral root; MBDR = medial branch; VA = vertebral artery
From Barnsley L, Bogduk N: Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Reg Anesth 1993;18:343-50
Function
The three-joint unit of the spine comprises the intervertebral disc and the paired facet joints sitting posterolaterally to the vertebral body. The orientation of the facet joints varies by and within the different portions (lumbar, thoracic, cervical) of the spine (Figure 5). The positioning and angles of the facet joints help to restrict motions. The greatest degree of motion and strain is at the two lowest facet joints (L4-5 and L5-S1). At these joints, strain is maximized by forward flexion and contralateral bending.[9]
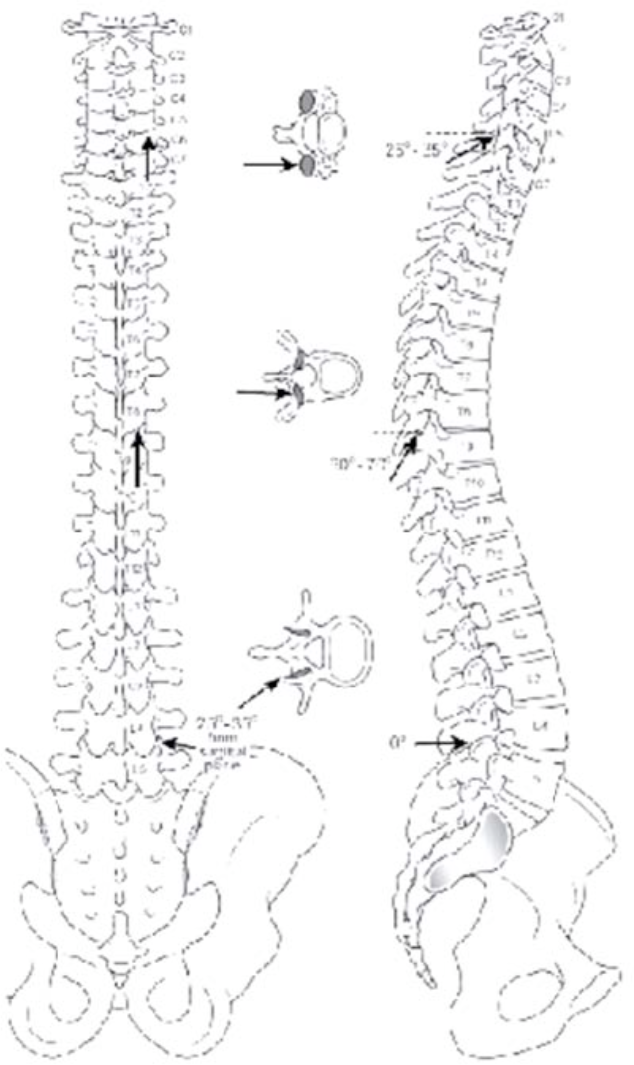
Figure 5. Facet Orientation. The lumbar facets in the parasagittal plane with the inferior articular process facing anterolaterally and the superior articular process facing posteromedially. The thoracic facet joints are the most vertically oriented. This positioning allows for lateral flexion without axial rotation. The cerfical facet joints have the greatest variation between the upper and lower joints. The laterla sketch shows the angle for needle entry to the intraarticular facet join at each level.
From Rathmell JP: Atlas of image-guided intervetion in regional anesthesia and pain medicine. Lippincott, Philadelphia, 2006. Figure 7-1, page 67
The greatest differences in orientation and function is in the cervical spine, with significant differences between the upper and lower joints.[10] The C2-3 joint, the most frequent cervical facet pain generator, is aligned approximately 70 degrees from the sagittal plane and 45 degrees from the axial plane, which inhibits rotation and anchors the C2 vertebra as a rotational pivot for the atlantoaxial joint (C1-2). At the level of C5-6, the cervical facets transition to a posteriorlateral orientation, making this the most mobile and second most affected cervical facet joint.
Pathophysiology
Facet arthropathy is most commonly associated with repetitive strain, intervertebral disc degeneration and minor trauma. In the cervical region, whiplash is a frequent cause of facet pain. In whiplash it is thought that excessive facet joint compression and capsular ligament strain occurs.[58] The facet joint and capsule are in close proximity to the semispinalis, multifidus, and rotator neck muscles thus it is thought that the excessive contraction of these muscles that occurs in whiplash contributes to their injury.[59-60] Whiplash injury and spine trauma are exceptions, especially in the cervical spine.[11-12] The association between the degree of inflammation or degeneration and pain is poor. Facet arthropathy is known to occur more commonly in the elderly, which is consistent with the concept of a degenerative disorder.[9] Fusion of an intervertebral level has been shown to accelerate degeneration at adjacent levels.[13] A hypertrophied facet joint osteophyte can compress a nerve root and cause radicular pain, especially in an intervertebral foramen that is already compressed due to disc protrusion.[14] Rarely, inflammatory arthritis and pseudocysts can cause facetogenic pain.
Prevalence
The true prevalence of facet pain is controversial. The diagnosis cannot be made by history, physical examination, or radiological findings, thereby making the incidence exceedingly difficult to determine. The most reliable method to determine facetogenic pain is with image-guided medial branch or intra-articular facet joint blocks.
The best estimates of lumbar facet pain in patients with LBP range between 10% and 15%.[5][15] Although comparative medial branch blocks (MBB) have been championed as a diagnostic standard, the other branches of the dorsal primary ramus will also invariably be blocked, which may overestimate the prevalence.[16] Another source of error is that most
epidemiological studies evaluating the prevalence of lumbar facet joint pain excluded patients with radicular symptoms, despite the fact that facet arthropathy can cause neuroforaminal stenosis.[14] There are few studies that have utilized placebo controlled MBBs (saline) to aid in the diagnosis.
Estimating the incidence of cervical facet pain is also difficult and is confounded by the multiple studies of patients with whiplash injury.[11-12] However, the best studies utilizing two diagnostic blocks have generally reported prevalence rates ranging between 49% and 60% in patients with chronic, non-radicular neck pain. Estimated prevalence varies between 40% and 50% in patients with chronic upper- and mid-back pain.
Diagnosis
History and Physical Examination
There are no history, physical examination, or radiological findings pathognomonic for diagnosis, but clinical assessment is important for ruling out other sources of pain and selecting candidates for interventions. The terms “lumbar facet syndrome” and “facet loading” were coined from a small, poorly designed retrospective study of 22 patients done in 1988.[17] Subsequent larger and methodologically sound studies failed to validate these findings.[9][18-19]
Pain referral patterns can provide clues for diagnosis (Figures 6 and 7a).
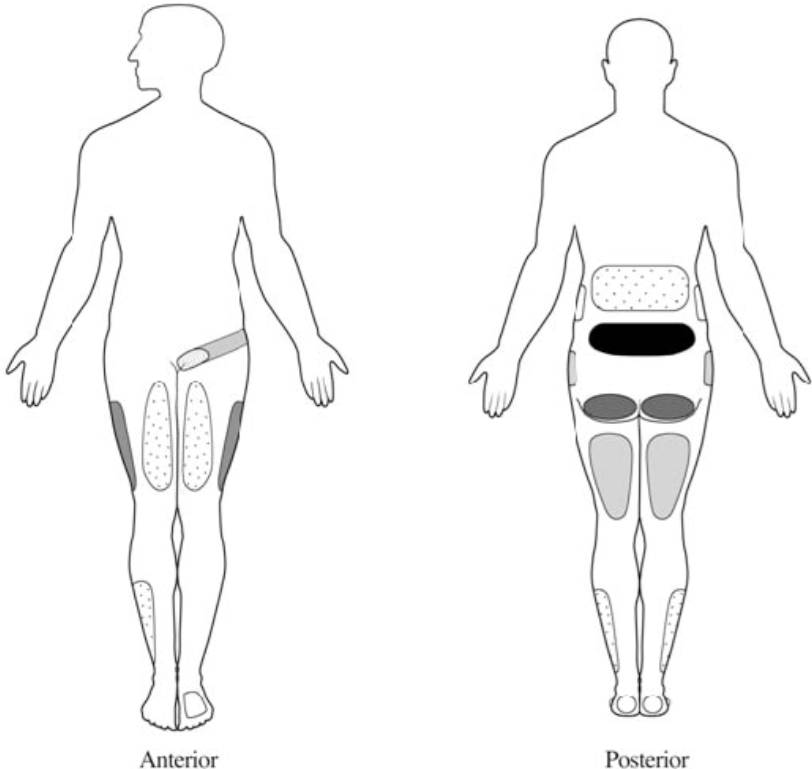
Figure 6. Lumbar facet pain referral patterns. The most common areas of referred pain from the lumbar facets are noted in black (low back) in descending order to the lightest regions (foot is least common). Each facet joint can refer pain to a number of locations, with a great deal of overlap between the different levels. Although there are trends, there are no direct associations between specific joints and referral pain regions.
From Cohen SP, Raja SN: Pathogenesis, diagnosis and treatment of lumbar zygapophysial [facet] joint pain. Anesthesiology 2007;106:591- 614.

Figure 7a. Cervical facet pain referral patters. The upper cervical facets are a common source of pain and headache, while pain from the lower cervical facets tends to be felt in the lower neck and trapezius region.
From Bogduk N, Marsland A: The cervical zygapophysial joints as a source of neck pain. Spine 1988;13:615.
Cervical facet clinical presentation
The most common symptom is unilateral pain that does not radiate past the shoulder. The pain is usually described as rather diffuse and is dull and aching in nature. It is usually not associated with motor or sensory deficit. Dwyer et al showed that injection of irritating substances into facet joints of normal participants resulted in specific referral patterns (see figure 7a)[61]
Lumbar facet clinical presentation
Pain is usually described as being in the lower back with possible radiation to the buttock, posterior thigh, groin, and rarely the lower extremity. It is described as achy in nature. (See figure 6 for referral patterns).
Thoracic facet clinical presentation
Pain is usually described as radiating anteriorly in a non-dermatomal distribution from the thoracic spine. It may be associated with thoracic paraspinal muscles spasm. (See figure 7b for referral patterns)
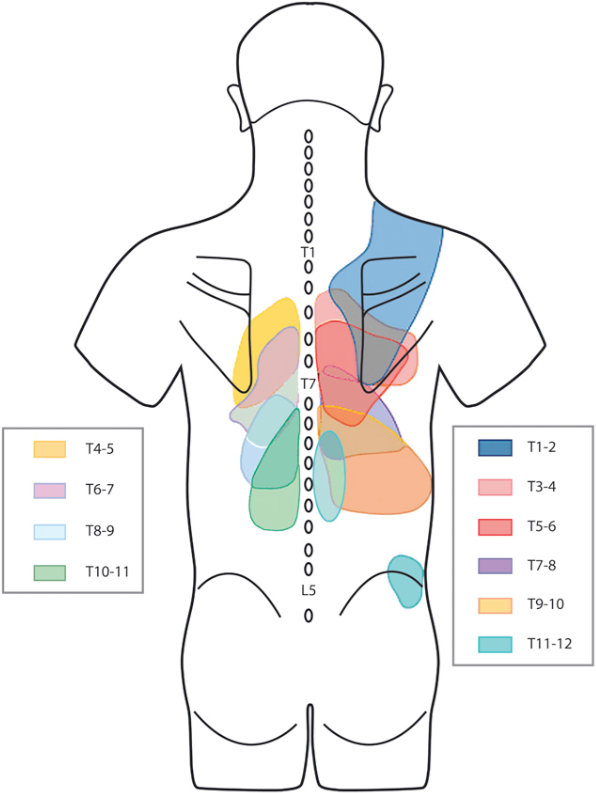
Figure 7b. Thoracic facet referral patterns. Thoracic facets tend to refer pain to the paraspinal regions around the thoracic spine. There tends to be significant overlap between the levels.
From Dreyfus P. et al: Thoracic zygaphophyseal joint pain patterns: a study in normal volunteers. Spine 1994; 19(7): 807-11.
Imaging Studies
Radiological examination has limited utility in the diagnosis of facet-mediated pain. The prevalence of facet pathology on computed tomography (CT) scans is between 40% and 85%, with the rate significantly increasing with age; however, the lumbar facet joints account for a much smaller percentage of chronic LBP cases.[20] Studies using MRI, CT and other imaging studies to predict response to facet blocks have been decidedly mixed, with most showing a poor correlation.[18] Cervical and lumbar facet pathology has been identified in 92% and 86% of cervical and lumbar SPECT/CT scans respectively. Of these pathological areas approximately 65% were identified as positive facet pain generators, however SPECT/CT is not routinely used clinically.[54]
Diagnostic Block
The inability to predict facet pain through history, physical, or radiological examination has led to the widespread use of medial branch and intra-articular facet blocks for diagnosis. Although diagnostic MBBs have been used to diagnose facet pain in multiple studies,21 they can be associated with false-positive and false-negative results.
Diagnostic Medial Branch Block
Lumbar
In the lumbar region, the needle is placed at the junction of the superior articular and transverse processes, where the medial branch passes. A separate injection can be made at each level (Figure 8a-c) or single needle skin entry at the middle level can be used to block three levels, thereby decreasing the number of skin entries and decreasing the superficial local anesthetic (LA) requirements (Figure 9). Small volumes (0.5 mL) of LA (0.5% bupivacaine, 1% lidocaine, 2% lidocaine, or 4% lidocaine) will suffice to provide short-lived blockade of the medial branch (2-4 hours).
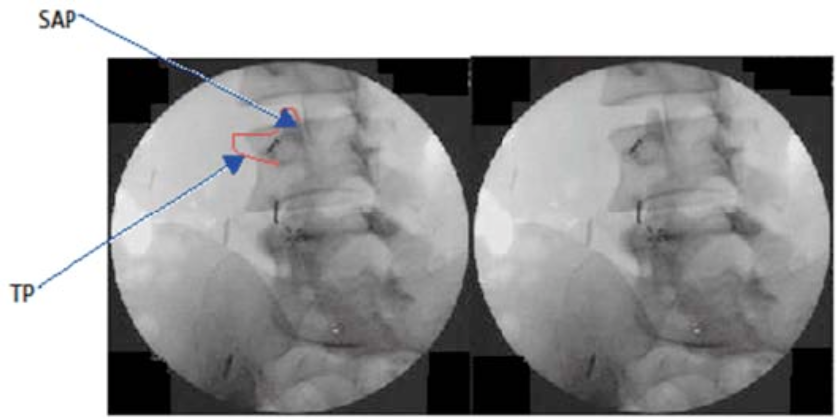
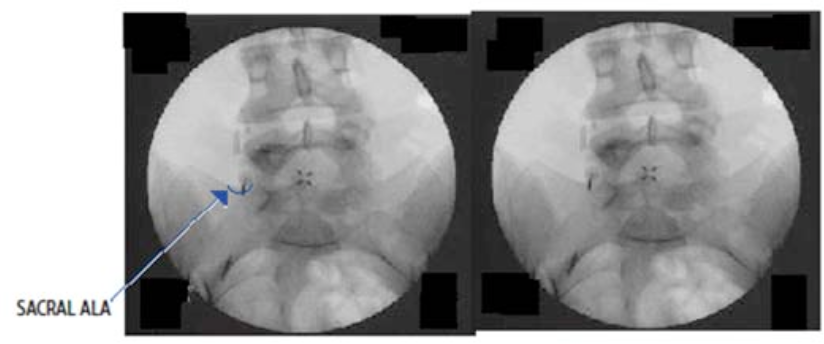
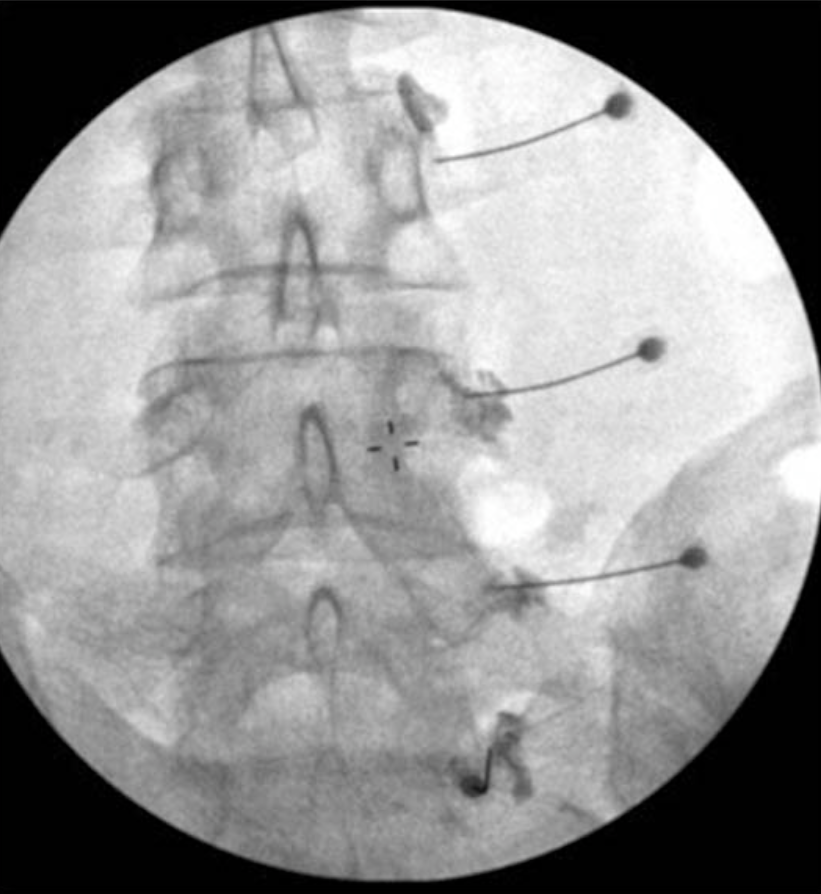
Figure 8 a-c. Diagnostic lumbar medial branch block. A 22-gauge spinal needle is placed at the junction of the superior (SAP) and transverse (TP) processes. (a) Oblique view demonstrates a 22-g needle in place near the left L3 and L4 medial branches at the junction of the SAP and TP. The left image shows the outline of the SAP and TP, while the right image is unaltered; (b) This AP image shows the needle placement at the sacral ala positioned to block the primary dorsal ramus of L5. The right image is the unaltered original; (c) This figure shows the contrast spread from an AP image of right-sided blocks of the L2, L3 and L4 medial branches and the primary dorsal ramus of L5.
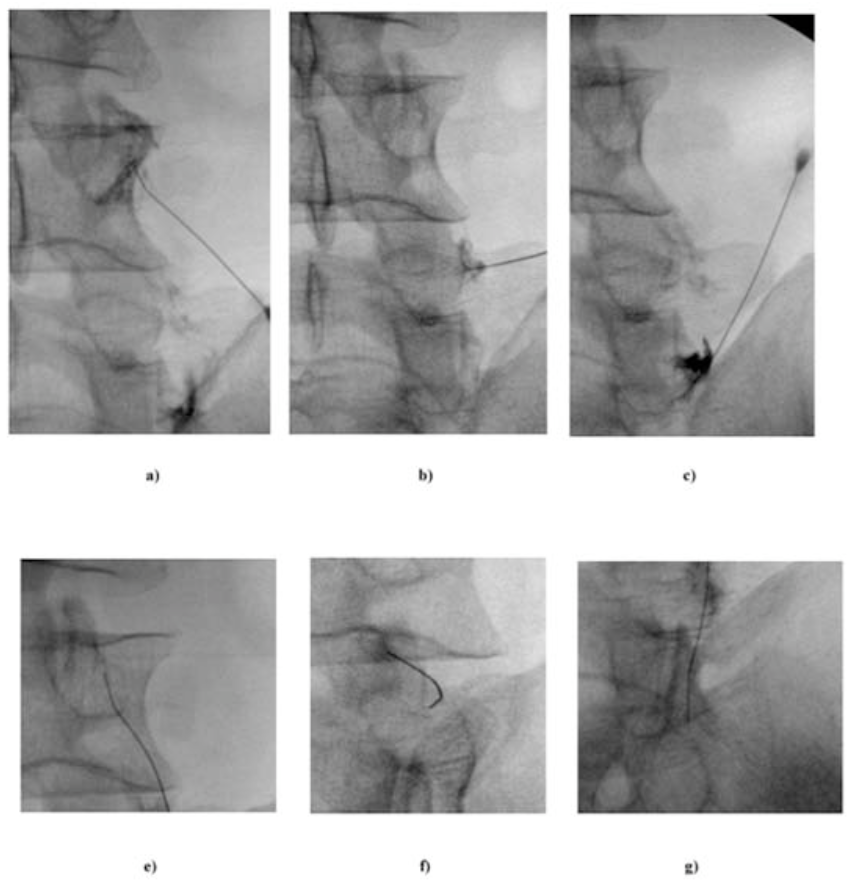 Figure 9. Diagnostic lumbar medial branch block via a single needle technique. Through a single needle skin entry site, three levels are blocked, thereby decreasing the number of skin entry sites and the amount of superficial local anesthetic used.
Figure 9. Diagnostic lumbar medial branch block via a single needle technique. Through a single needle skin entry site, three levels are blocked, thereby decreasing the number of skin entry sites and the amount of superficial local anesthetic used.
From Stojanovic MP, Zhou Y, Hord ED, et al: Single needle approach for multiple medial branch blocks: a new technique. Clin J Pain 2003;19:134-7.
Cervical
Diagnostic blockade of the cervical spine is accomplished by placing a small volume of LA (0.3-0.5 mL) at the waist of the articular pillar. This can be accomplished by coming from the lateral neck with the patient positioning either supine or lateral (Figures 4 and 10), or the patient can positioned prone and a posterior to anterior approach can be employed (Figures 11 a-b and 12 a-b). In some cases, the duration of pain relief may exceed the expected duration of LA, especially in the case of upper cervical facet blocks for headaches.
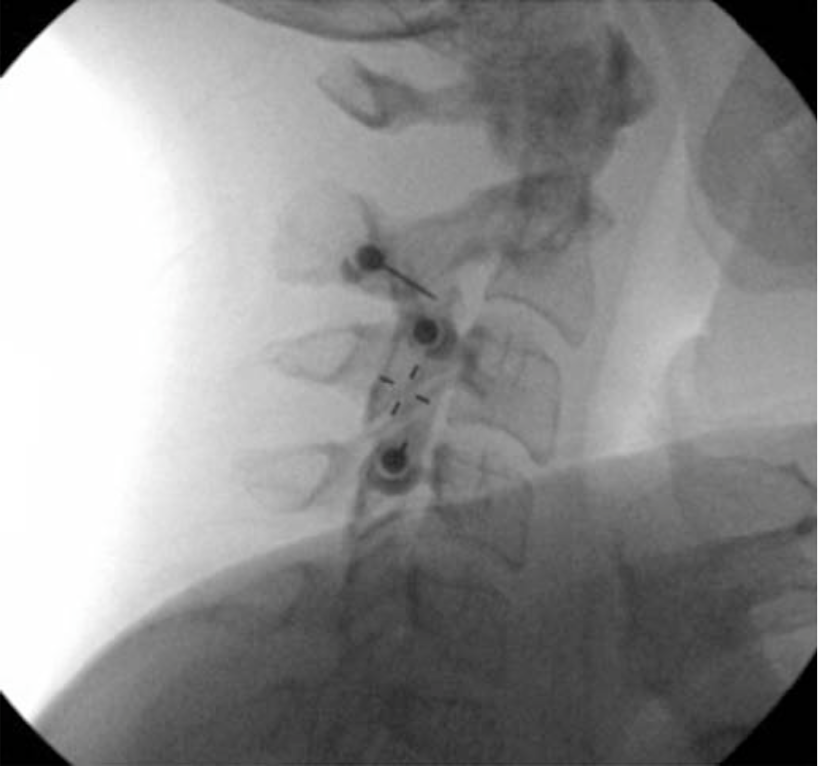 | 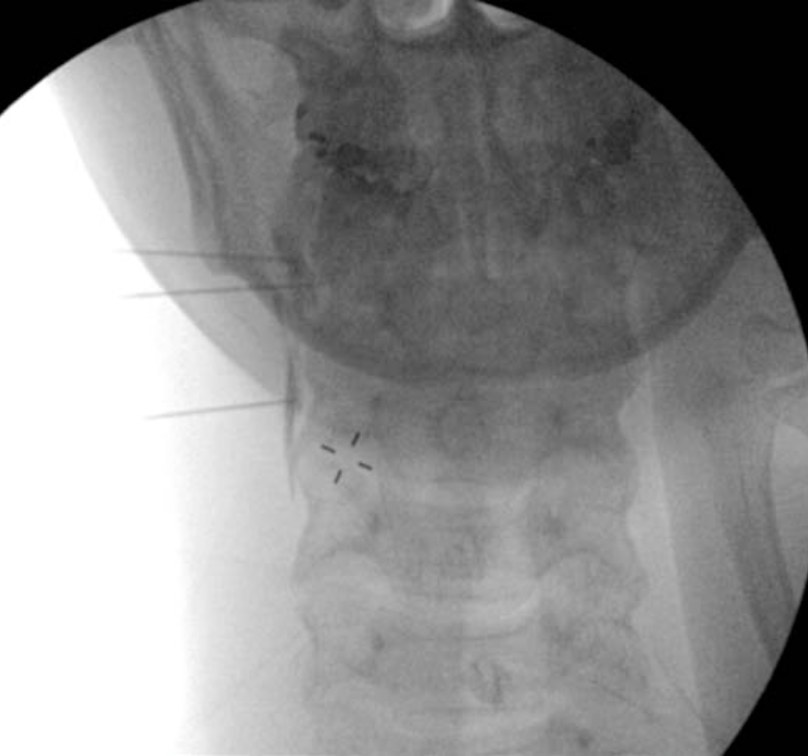 |
Figure 10 a-b. Lateral approach for a cervical medial branch block. With the patient positioned either supine or lateral, the skin over that lateral neck is anesthetize d and a spinal needle is directed to the target point at the waist of the cervical articular pillar. The needles are directed from the lateral neck towards the waist portion of the articular process (10a) and confirmed using an AP image (10b). The top needle in this figure is directed at the junction of C2 and C3 where the third occipital nerve (TON) lies. The AP image demonstrates contrast spread.
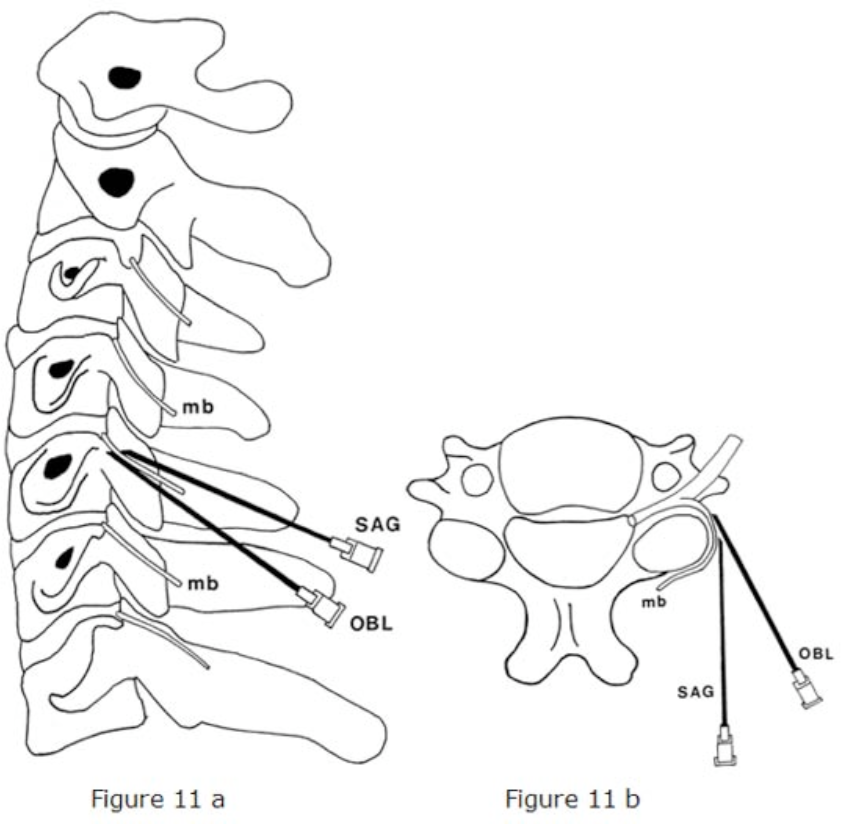
Figure 11 a-b. Posterior approach for cervical medial branch block and denervation. A spinal or radiofrequency needle can be directed from posterior to anterior to the waist of the articular pillar to block or denervate the cervical medial branch. mb = medial branch; OBL = oblique approach; SAG = sagittal approach
From McDonald GJ, Lord SM, Bogduk N: Long- term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery 1999;45:61-7.
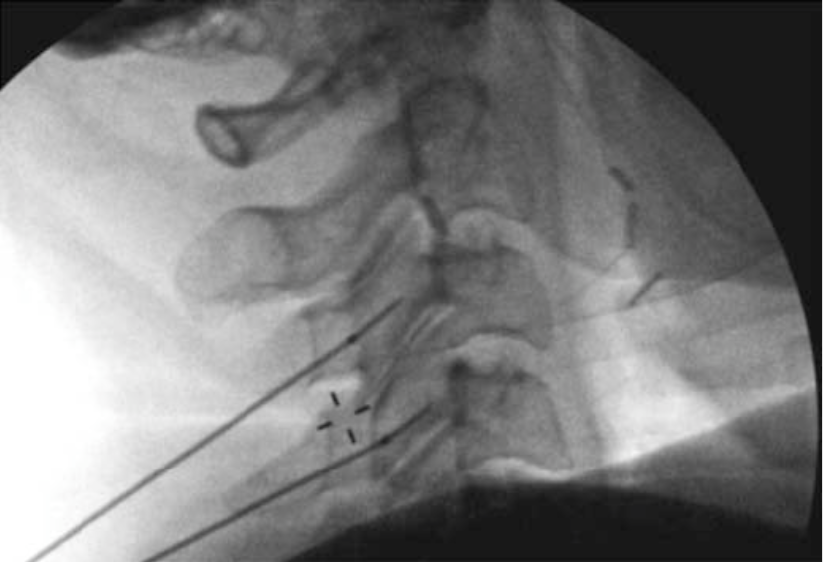 | 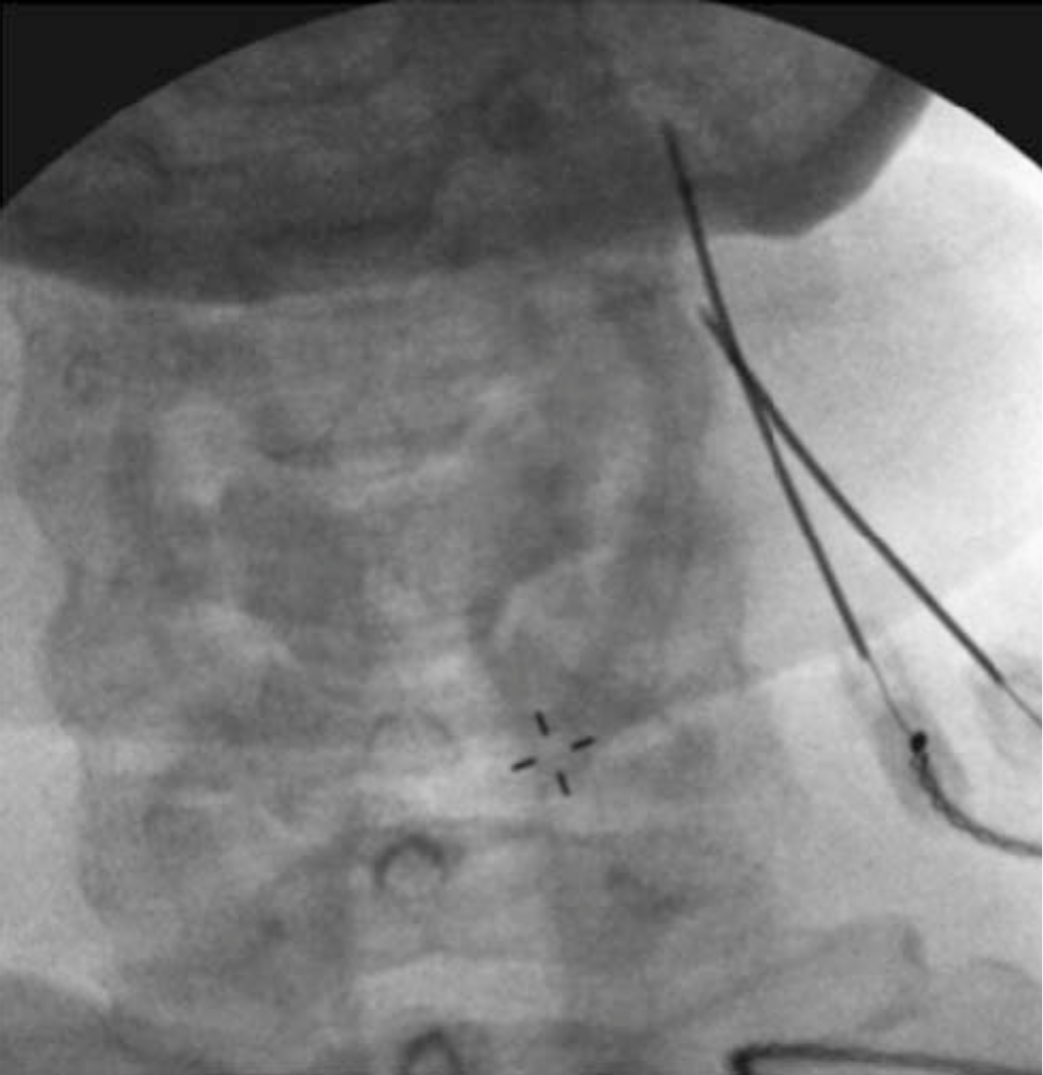 |
Figure 12b. For a more detailed explanation of the technique of MBB, we recommend the Atlas of Image-Guided Regional Anesthesia and Pain Medicine by James P. Rathmell[22] and the International Spine Intervention Society: Practice Guidelines-Spinal Diagnostic and Treatment Procedures.[23
Figure 12 a-b. Posterior approach for a cervical medial branch denervation. With the patient positioned prone, the needle is directed from posterior to anterior along the lateral portion of the cervical spine to the waist of the articular pillar. Needle position is confirmed using lateral (a) and AP (b) images. The posterior approach for blockade of the cervical medial branch is similar.
Thoracic
Diagnostic blocks in the thoracic region are performed by placing the needle at the superior lateral corner of the transverse process where the medial branch transverses via a posterior approach. These targets are especially true for medial branches T1-4 and T9-T10. (Figure 13). A separate injection can be made at each level. A small amount of local anesthetic (0.5 ml of 0.5% bupivacaine or 2% lidocaine) should be injected at each level. It is important to keep the needle over bone at all times to avoid damage to the pleura. It is also important to note that there is greater variability at the levels of the T5-T8. At these levels the medial branches are often found in the intertransverse spaces rather than on bone. This fact can lead to difficult when performing the procedure at these levels.
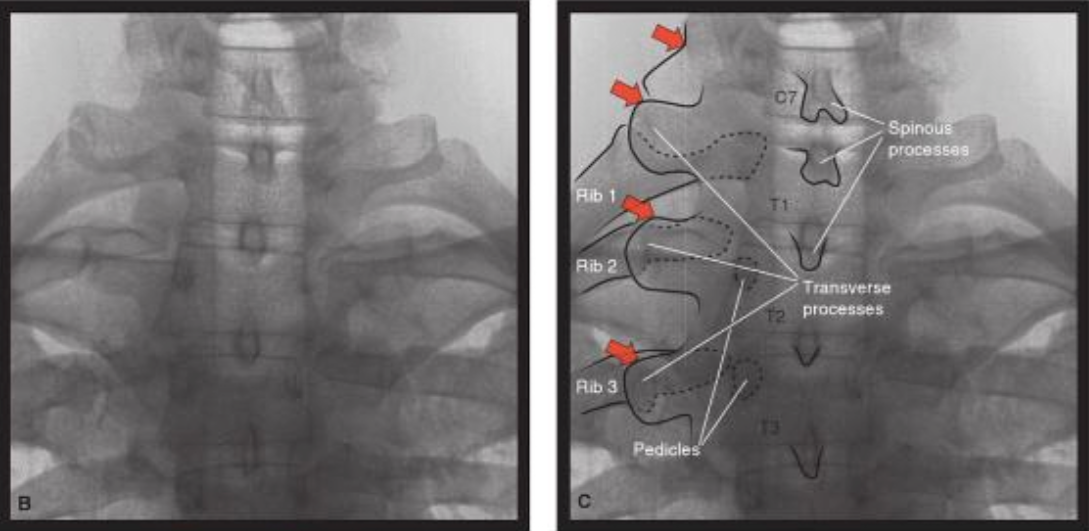
Figure 13a. Upper thoracic medial branch nerve targets (AP view). The transverse processes are usually prominent at the upper thoracic levels. Red arrows indicate the target position for the medial branch nerves at vertebral levels C7-T3.
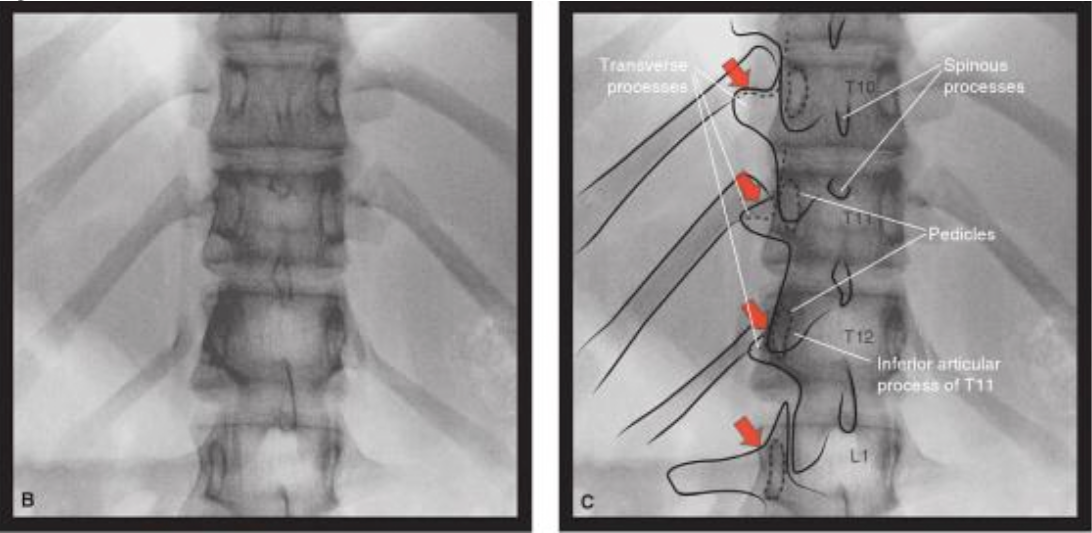
Figure 13b. Lower thoracic medial branch nerve targets (AP view). The transverse processes may be more difficult to identify at the lower thoracic levels. The red arrows indicate the target points of the medial branch nerves at vertebral levels for T10-L1. The target position for T11 and T12 is at the junction of the superior articular process and the transverse process similar to the target for the lumbar medial branches
Used with permission from Rathmell J. Atlas of Image–Guided Intervention in Regional Anesthesia and Pain Medicine 2nd Ed. Philadelphia, Lippincott Williams and Wilkins 2012: pages 108-109 (figures 7-32, 7-33.
Diagnostic Intra-articular Facet Block
Intra-articular facet blocks with local anesthetic are another type of diagnostic block.
While some pain physicians use intra-articular blocks for both diagnostic and therapeutic purposes (through the addition of steroid), the long-term analgesic benefit is questionable. A short-term analgesic benefit from intra-articular blocks can be viewed as a similar diagnostic tool to MBBs. There are less data available to determine the false-positive and –negative responses to intra-articular facet joint injections. For further discussion of intra-articular facet blocks, see the Treatment section below.
Interpretation of Diagnostic Block Results
False Positive Diagnostic Block
False positive rates for diagnostic MBBs have ranged from 25%–40% in the lumbar spine9 to 25%–30% in the cervical spine.[24-25] Some recommend comparative LA blocks with lidocaine and bupivacaine to aid in diagnosis; however, this has been shown to be highly sensitive (88%) but only marginally specific (54%).[26] Placebo-controlled blocks (normal saline) offer the best sensitivity and specificity, but saline blocks are not an ethical option outside of the research realm.
For diagnostic medial branch block, small volumes of LA can spread over a wide area, with 0.5 mL known to cover 6 cm2 of tissue.[9] This wide spread of LA can block afferent transmission of the paraspinous muscles and sacroiliac joint by blocking the lateral branch. Cohen et al.[27] recently demonstrated that the reduction of LA volume for diagnostic cervical MBBs from 0.5 mL to 0.25 mL improved specificity without adversely altering the sensitivity.
The use of intra-articular facet injections can reduce issues related to the inadvertent spread of LA to other structures but can be technically challenging. Furthermore, excessive volumes of LA solution can rupture the joint capsule, leading to spread into the intervertebral foramen epidural space, and paraspinous musculature.[21][28-29] Although it is often written that MBBs provide comparable relief and diagnostic utility to intraarticular injections, the lack of any randomized crossover studies preclude definitive conclusions from being drawn.
Comparisons between diagnostic MBBs and intra-articular injections are summarized in Table 1.
| Type of Injection | Medial Branch Block | Intra-articular Facet Block |
|---|---|---|
| LA = local anesthetic; RF = radiofrequency | ||
| Target | Medial branch only | Intra-articular injection |
| Typical Injection Volume | 0.25-0.5 mL of LA | 1-1.5 mL LA + steroid (lumbar) 0.5-1.0 mL LA + steroid (cervical) |
| Expected Response | Short (hours) | Varies, but intended to be long (days to weeks) |
| False Positives | Common | ? |
| False Negatives | Vascular uptake | ? |
| Advantages | Aids diagnosis when stringent criteria are used Can be followed by RF, which can provide pain relief for 6-12 months | Simple No need to buy RF equipment |
| Disadvantages | Need RF equipment Complications associated with RF | Can be challenging at times due to distorted anatomy Need for repeat injections |
Potential causes of false-positive blocks include placebo response, sedation, excessive superficial local anesthesia, and the spread of local anesthetic to other pain-generating structures.[30] Recommendations to limit false positive blocks are listed in Table 2.
Table 2. Techniques to Reduce the False-Positive Rates for Lumbar Medial Branch Blocks
- Avoid the use of sedation and analgesics.
- Use injectate volumes of ≤ 0.5 mL.
- Limit volume of local anesthesia.
- Aim for lower target point on transverse process.
- Use a single-needle approach.
- Consider use of comparative local anesthetic blocks.
False Negative Diagnostic Block
False-negative blocks have received less attention than false-positives, but they can still be an important source of misdiagnosis. One of the principal causes of false-negative blocks is believed to be vascular uptake, which has been reported to range between 6% and 30% per level.[31-32] Aberrant innervation has been described as a potential cause. The most reliable means to detect vascular uptake is with real-time fluoroscopy using contrast dye;[31] however needle repositioning after noted intravascular uptake may still fail to provide analgesia.[32] Not all practitioners recommend the use of dye during diagnostic blocks. Other potential causes of false-negative blocks are failure to discern between baseline and procedure-related pain and missing a target nerve(s).
Repeated Diagnostic Blocks
The issue of how many blocks should be performed is also the subject of considerable controversy. While many argue that comparative LA blocks (bupivacaine and lidocaine at separate time points) or repeated diagnostic blocks are critical for an accurate diagnosis, multiple retrospective studies evaluating outcomes for lumbar facet, cervical facet and sacroiliac joint RF denervation have failed to find a difference in success rates between patients selected with one and two diagnostic blocks.[33] The cost and time associated with double-diagnostic blocks may not be practical, and there is no evidence to support a longer duration of action for a bupivacaine MBB when compared with a lidocaine MBB.
In addition, many physicians apply loose standards to the results of double-diagnostic blocks by continuing to RF in patients failing to respond to a second block or sometimes performing a third diagnostic block. The low morbidity and mortality of RF denervation calls into question such rigorous diagnostic screening. The best indication for repeated diagnostic MBB is the suspected false-negative block due to intravascular uptake or other potential failure.
Treatment
Pharmacological and Non Interventional Therapy
The growth of minimally invasive spine therapies over the last decade has heavily shifted the therapy of spine pain towards interventions, while conservative therapy still has a role alone or in conjunction with interventions. Although the effect size is small, non-steroidals and acetaminophen can be effective. Most types of physical activity will be beneficial in back pain, including structured physical therapy, light exercise, stretching, and yoga. Spinal manipulation is superior to sham treatment for acute and chronic spinal pain, but the long- term benefits remain to be proven. Acupuncture also appears effective for spinal pain, but has not been shown to be superior to other treatments. Psychotherapy can aid in the treatment of patients with comorbid depression or anxiety, as untreated psychopathology has been noted to predict poorer outcomes.
Interventional Procedures - TOP
Intra-articular Corticosteroid Injection
Many physicians prefer intra-articular injections with local anesthetic and steroid in lieu of MBBs due to the belief that they provide both diagnostic and therapeutic benefit. In the best- designed studies, no sustained benefit with intra-articular steroid injections has been demonstrated.[34-35] However, several uncontrolled studies have found that patients with an active inflammatory process, as demonstrated by positive SPECT scans, may demonstrate intermediate-term relief.[9] Given that facetogenic pain is mainly due to chronic degeneration rather than acute inflammation, the lack of response to intraarticular steroids is not surprising.
Therapeutic Medial Branch Block
There have been a number of studies describing the use of repeated MBBs with LA alone, LA plus steroid, and LA plus other additives (Sarapin). Although some authors have championed the use of MBBs as a therapeutic option,[36] more scientifically rigorous studies have not validated these findings.[21] Therefore, we do not recommend repeated MBBs or the use of steroid with LA for MBBs for treatment.
Radiofrequency (RF) Denervation of the Medial Branch (Thermal)
The most commonly performed treatment for facet-mediated pain is RF denervation. There have been several randomized control trials for facet pain.[12][37-43] There have been methodological faults with some of the studies, including failure to select patients using diagnostic blocks and suboptimal RF needle placement (placed perpendicular rather than parallel to the nerve). In all of the studies, diagnostic blocks were first performed. There is however, no consensus on how to perform diagnostic blocks, thereby resulting in different techniques used, different medications, different doses of the same medication, and different selection criteria. These differences may result in the different success rates found in the different randomized controlled trials.[63] The overall conclusion when considering these studies favors RF denervation in well-selected patients.
The appropriate patient-reported pain relief necessary to recommend subsequent denervation has been debated. Some studies recommend a 2-point or 30% decrease in pain to be clinically meaningful[44] while other studies have found no differences in radiofrequency (RF) outcomes when using 50% versus 80% pain relief as the measure for denervation.[18-19][33] Due to the multifactorial nature of back and neck pain, few patients describe complete relief after diagnostic MBBs or RF denervation.
RF denervation of the medial branch is accomplished by placing the active portion of the electrode at the location of the nerve, ideally with the length of the active electrode parallel with the nerve to allow for lesioning of a longer portion of the nerve (Figure 14).
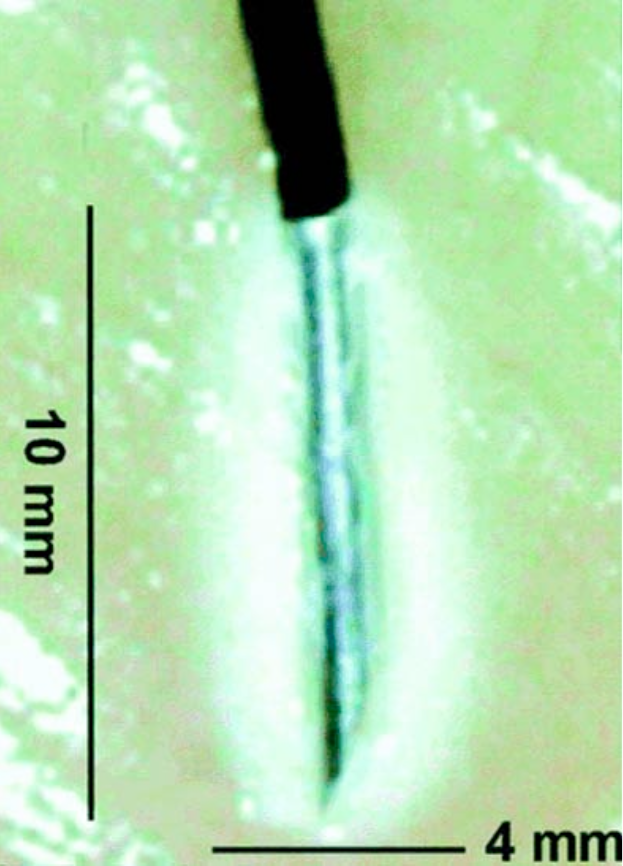
Figure 14. Radiofrequency lesion. For radiofrequency lesioning, the 10-mm active tip of the radiofrequency needle should be placed parallel to the nerve to create the largest lesion possible. When the needle is placed parallel to the nerve, the lesion size will be at least 10 mm in length, wheras the same needle placed perpendicular to the nerve would create a legion less than 4 mm in length
Adapted from Cohen Sp, Hurley RW, Buckenmaier CC, 3rd, et all: Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac join pain. Anesthesiology 2008; 109:279–88.
Lumbar
For the lumbar region, the active electrode is optimally positioned at the junction of the transverse process and lateral neck of the superior articular process in an orientation parallel to the nerve (Figure 15 a–c).
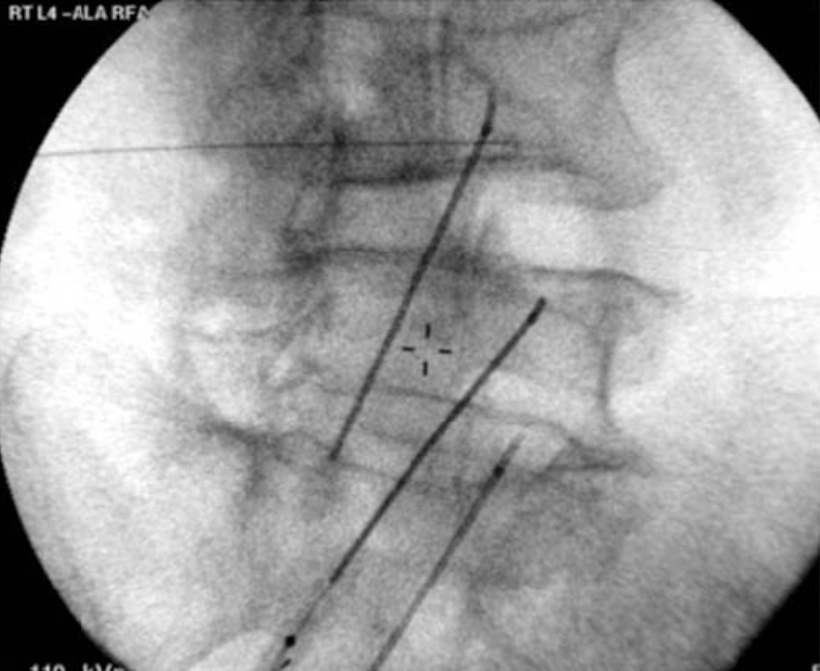 | 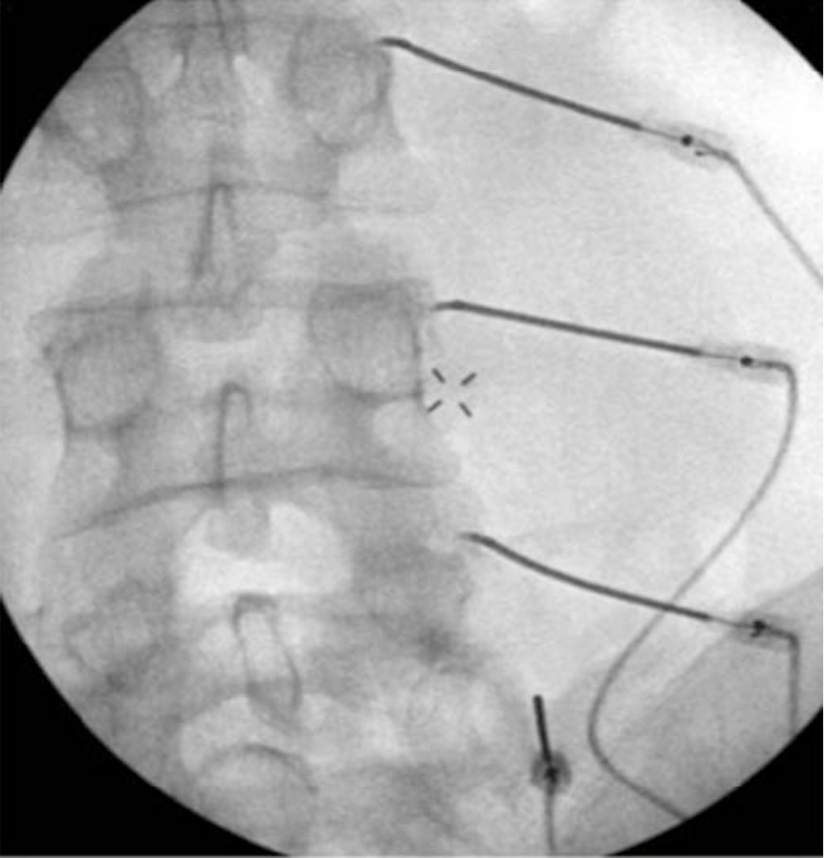 | 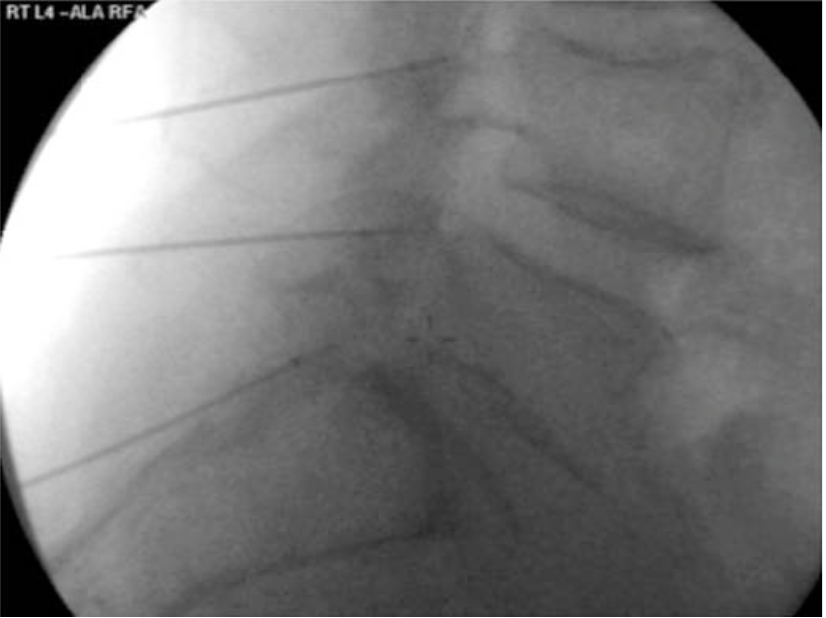 |
Figure 15 a-c. Lumbar medial branch radiofrequency denervation. The active portion of the electrode is placed parallel to the medial branch along the lateral neck of the superior articular process at the junction with the transverse process. The needle is directed to the target site via an oblique approach (a). AP (b) and lateral (c) images can sometimes assist in appropriate needle placement. Lateral images are generally most useful when there is concern that the needle may be placed too far anterior, thereby putting the nerve root at risk for damage during denervation.
Cervical
In the cervical region, the active tip should be placed along the center of the articular pillar at most levels, similar to the needle placement for a posterior approach for a diagnostic cervical MBB (Figure 12 a-b).
Thoracic
In the thoracic region, the active tip should be placed at the superior lateral corner of the transverse process in a parallel orientation to the nerve.
Sensory stimulation is usually performed prior to denervation, with most experts recommending a threshold ≤ 0.5 volts. Cohen et al, however did not actually find an association between lower sensory stimulation thresholds during sensory testing and positive treatment outcomes provided that sensory thresholds were optimized.[66] Motor stimulation is considered a safety measure to ensure adequate distance from motor fibers, although the elicitation of multifidus muscle contraction has also been used to guide needle placement.45 Prior to denervation, local anesthetic with or without steroid can be injected to reduce procedure-related pain, enhance lesion size, and prevent neuritis. Diclofenac sodium has also been shown to enhance patient satisfaction and reduce the risk of neuritis following conventional RF denervation. A 3 day course of diclofenac sodium has been found to be sufficient.[55] Most studies describe between 6 months and 1-year of relief following RF denervation.[12][46] The procedure can be repeated when the pain returns. Repeat denervation appears to provide comparable relief to the initial procedure.[47] A different study assessed the effects of one, two, and three radiofrequency treatments found that patients who had initially responded also had a positive response to the 2nd and 3rd intervention. The duration of relief was found to be about 10 months and this did not change with repeat procedures.[64]
For a more detailed explanation of the technique of lumbar and cervical RF denervation, we recommend the Atlas of Image-Guided Regional Anesthesia and Pain Medicine by James P. Rathmell[22] and the International Spine Intervention Society: Practice Guidelines-Spinal Diagnostic and Treatment Procedures.[23]
COOLED Radiofrequency Denervation
Water cooled RF ablation is an alternative technique for ablation of the medial branch nerves. The basic principle is similar to traditional RF, however, in contrast to traditional RF the volume of the tissue heated is larger and thus the resultant thermal lesion is larger than in traditional RF. Theoretically, the larger area of neurodestruction increases the probability of a successful denervation given the variable nature of the medial branch nerves. In addition, the technique may be more easily performed in that it allows for a perpendicular approach to the nerves rather than the parallel approach taken in traditional RF. This perpendicular approach can be taken because in Cooled RF more energy is dissipated off of the tip of the needle as compared to traditional RF (Figure 16).
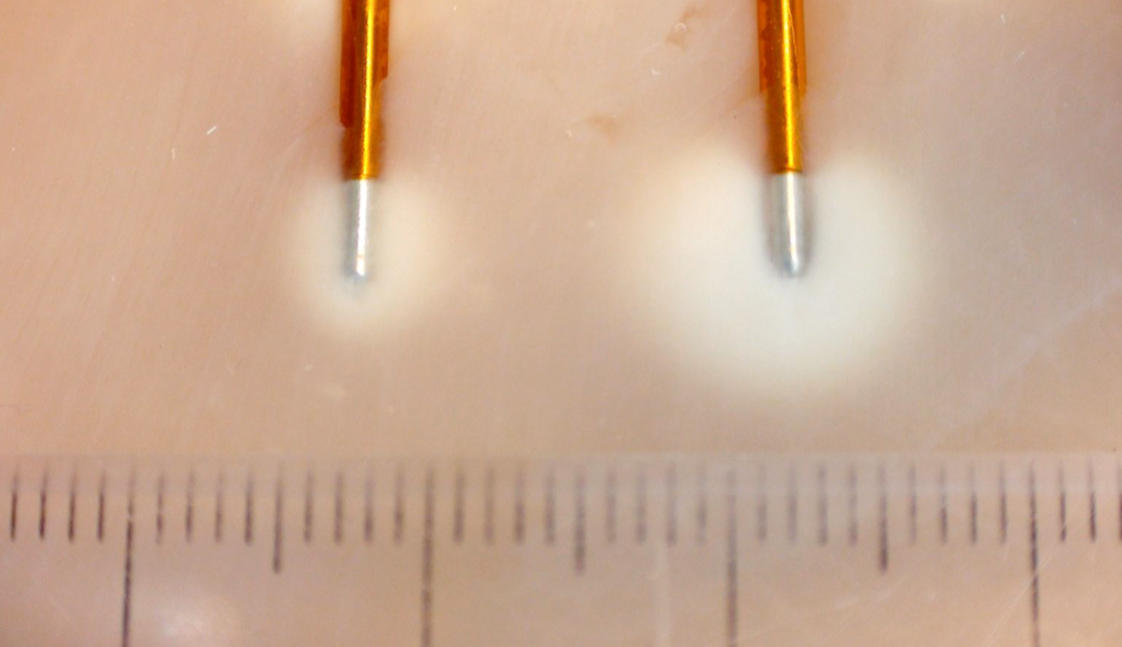
Figure 16. Physics of Cooled RF. Probe on left depicts size of radiofrequency lesion using traditional RF. Probe on right depicts lesion size of radiofrequency lesion using Cooed RF
Used with permission from Kimberly Clark
Cervical Cooled RF procedure
Can be done by either the prone or lateral approach. A 17 Gauge 50mm or 75mm needle with a 2mm active tip is used. A lesion size of 4-6 mm will be created. As more energy is dispersed out from the tip of the needle a perpendicular approach to the nerve can be taken. The target points are the same as with traditional RF (the waists/center of the articular pillars) (Figure 17a-b). Sensory stimulation is not necessary with the use of Cooled RF as the lesion size is thought to be large enough to encompass the variable anatomy of the medial branch nerve. One may perform sensory testing however if desired. Motor stimulation is still considered a safety measure to ensure adequate distance from motor fibers. Prior to denervation local anesthetic (0.3 to 0.5 ml of 0.5% bupivacaine or 2% lidocaine) with or without steroid can be added to reduce discomfort and prevent neuritis. Lesioning then takes place at 60 degrees C for 150 seconds.
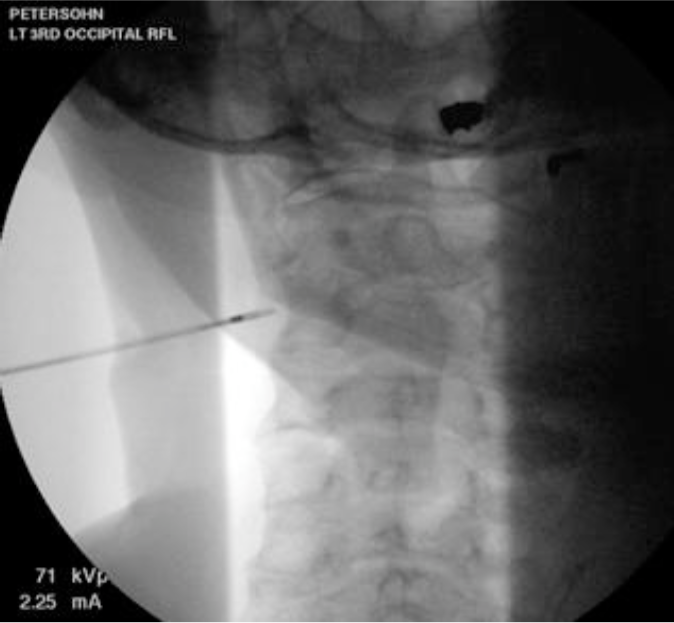
Figure: 17a. Cervical Cooled RF procedure. AP view of needle approaching the C3 Medial branch nerve at the waist of the articular process.
Permission to publish granted from Kimberly Clark
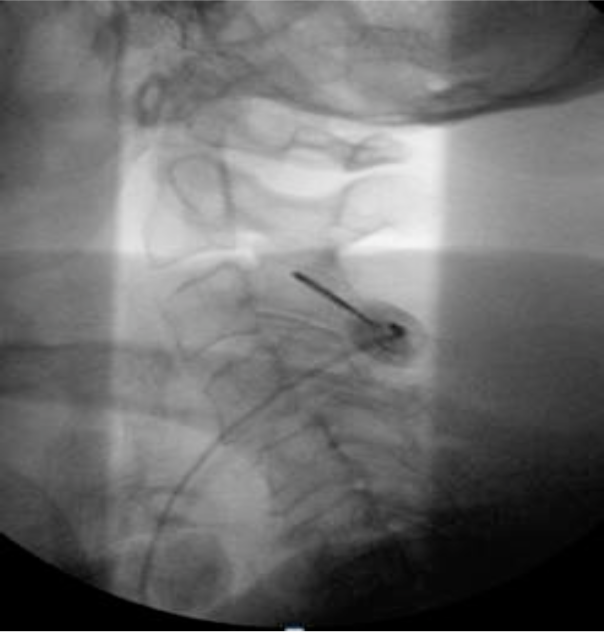 Figure 17b. Cervical Cooled RF procedure. Lateral view of the needle at its target on the C3 articular pillar
Figure 17b. Cervical Cooled RF procedure. Lateral view of the needle at its target on the C3 articular pillar
Permission to publish granted from Kimberly Clark
Thoracic Cooled RF procedure
Done via a posterior approach. The needle used is a 17G 75mm with a 5.5mm active tip. The lesion size is estimated to be 12 mm in size. This lesion size allows for ablation of nerves both on the bony surface and those in the intertransverse space. A skin insertion site is identified slightly lateral to the target site to ensure safety (lessen the risk to the lungs). The needle is advanced to the superior lateral corner of the transverse process and then walked off slightly. (Figures 18a-d)
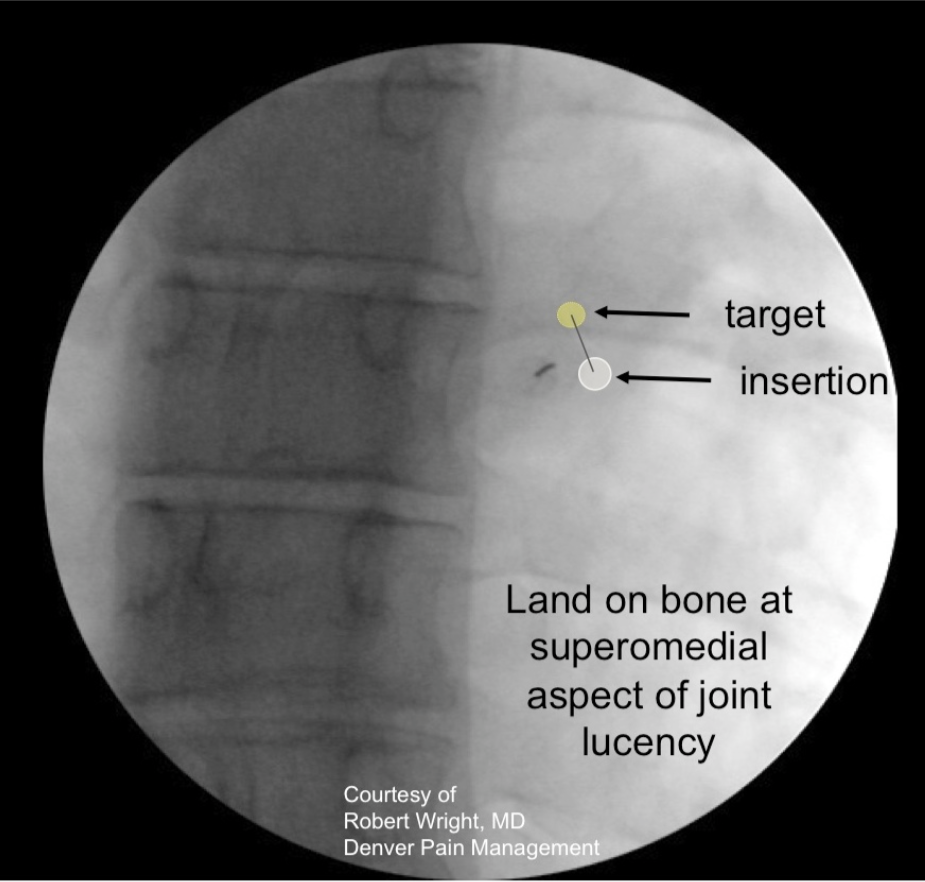
Figure 18a. Thoracic Cooled RF medial branch denervation procedure. Position of skin insertion point just inferior and lateral to the superior lateral edge of the transverse process in a slight ipsilateral oblique view. The initial target position is on the edge of the superiormedial aspect of the joint lucency.
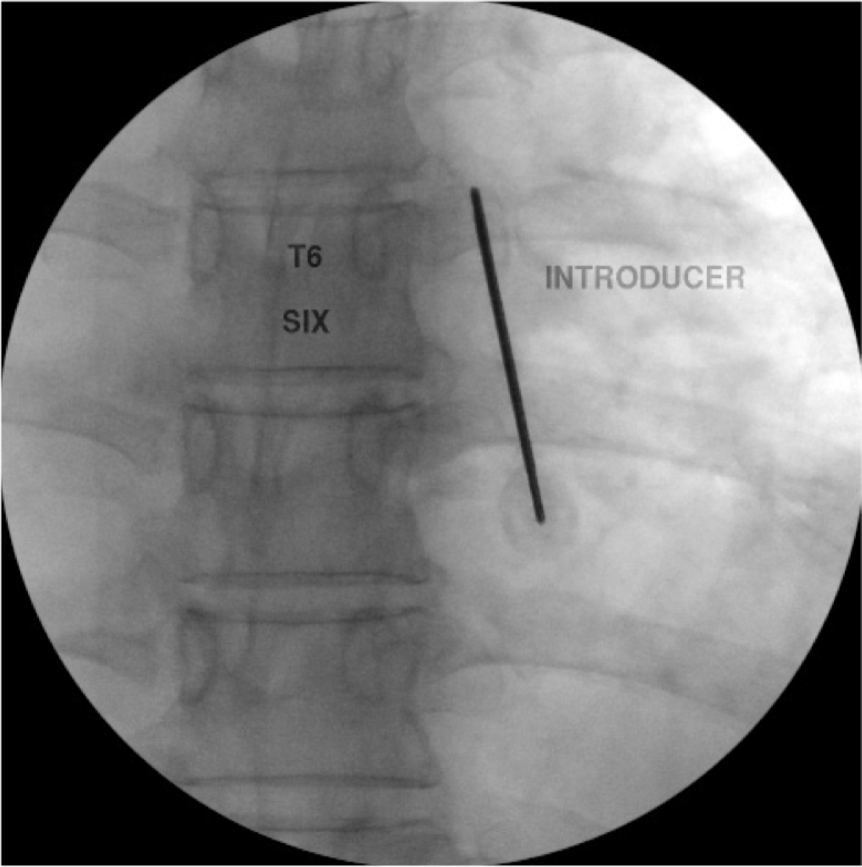
Figure 18b. Thoracic Cooled RF medial branch denervation procedure. The needle is then walked to the superior margin of the transverse process in the AP view.
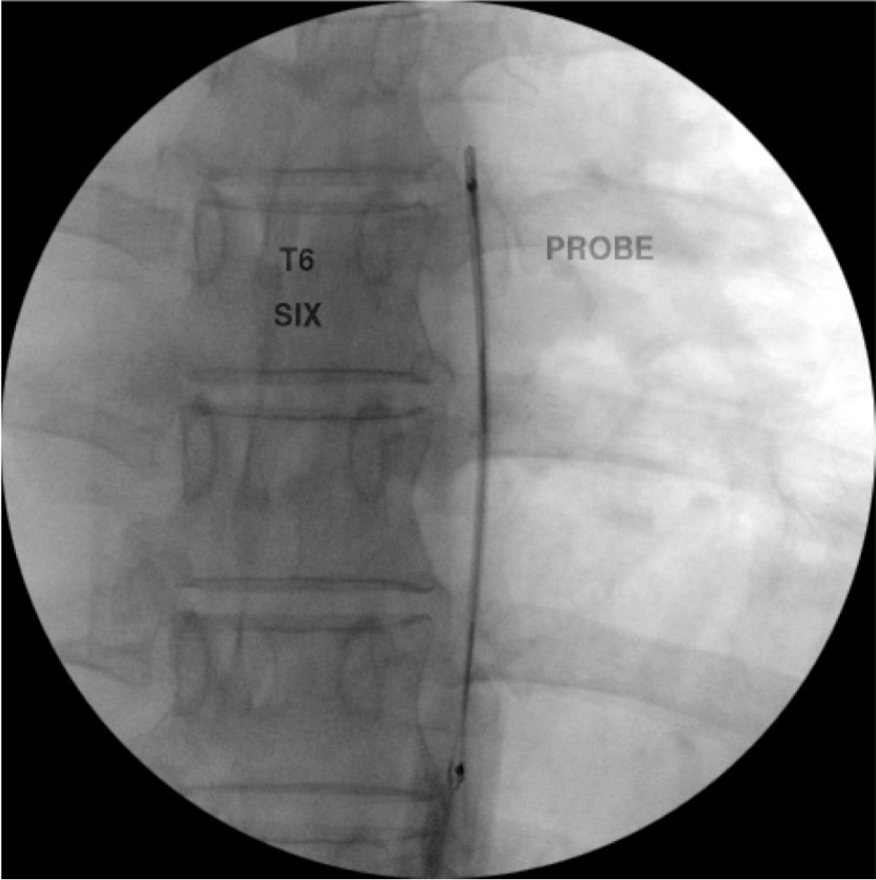
Figure 18c. Thoracic medial branch denervation procedure. The RF probe has been inserted into the RF needle and is in position just off the superior lateral edge of the T6 transverse process.
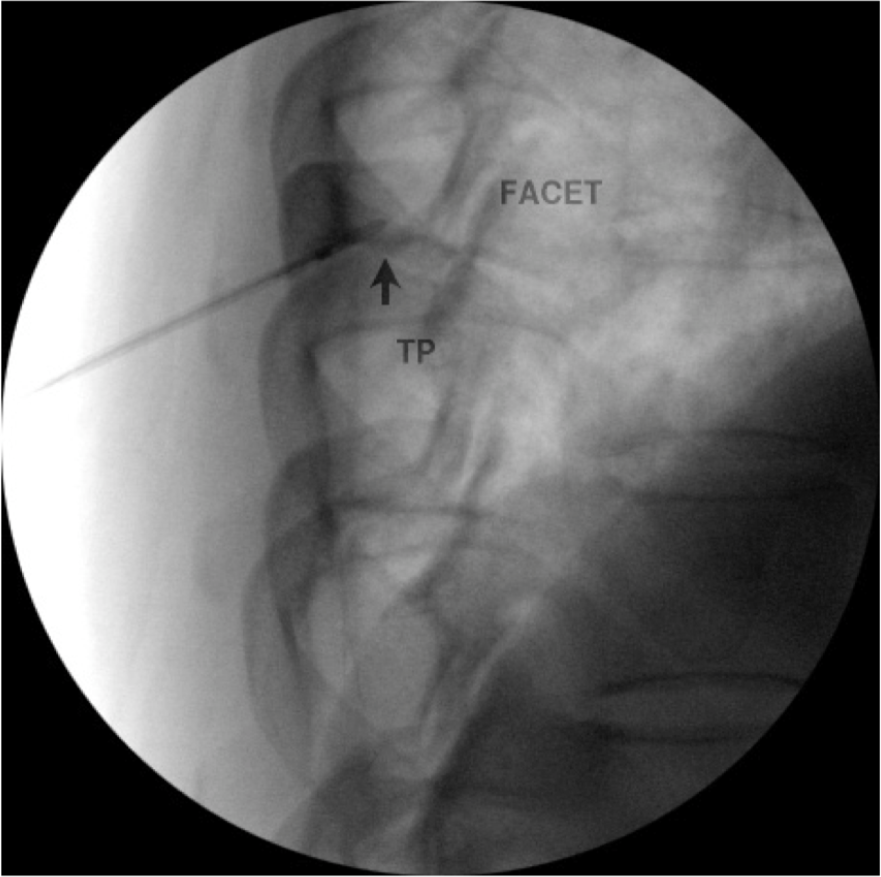
Figure 18d. Thoracic medial branch denervation procedure. Lateral view of probe in position posterior to the foramen.
A small amount of local anesthetic (0.5 ml of 0.5% bupivacaine or 2% lidocaine) is given with or without steroid to reduce discomfort and/or neuritis. Lesioning takes place at 60 degrees C for 150 seconds.
Lumbar Cooled RF procedure
Procedure is performed in the prone position. An AP view is first taken to square of the vertebral endplates. Next the c-arm is rotated ipsilaterally until the SAP is in the center of the disk. The target is the midpoint of the lateral surface of the SAP. A lateral view is then taken to ensure safe depth away from the neural foramen. (Figures 19a-c) A small dose of local anesthetic (0.5 ml of 0.5 % bupivacaine or 2% lidocaine) with or without steroid is given to reduce discomfort and/or neuritis. Lesioning takes place at 60 degrees C for 150 seconds.
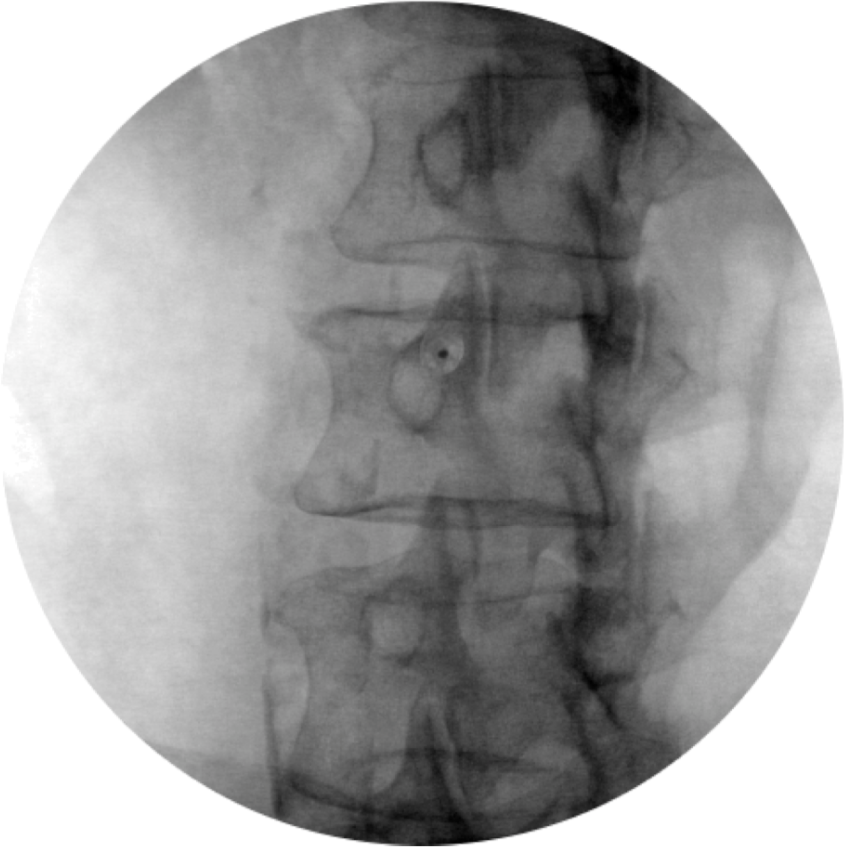
Figure 19a. Lumbar medial branch denervation procedure. Oblique view of needle place on target at base of SAP. (permission to publish granted from Kimberly Clark)
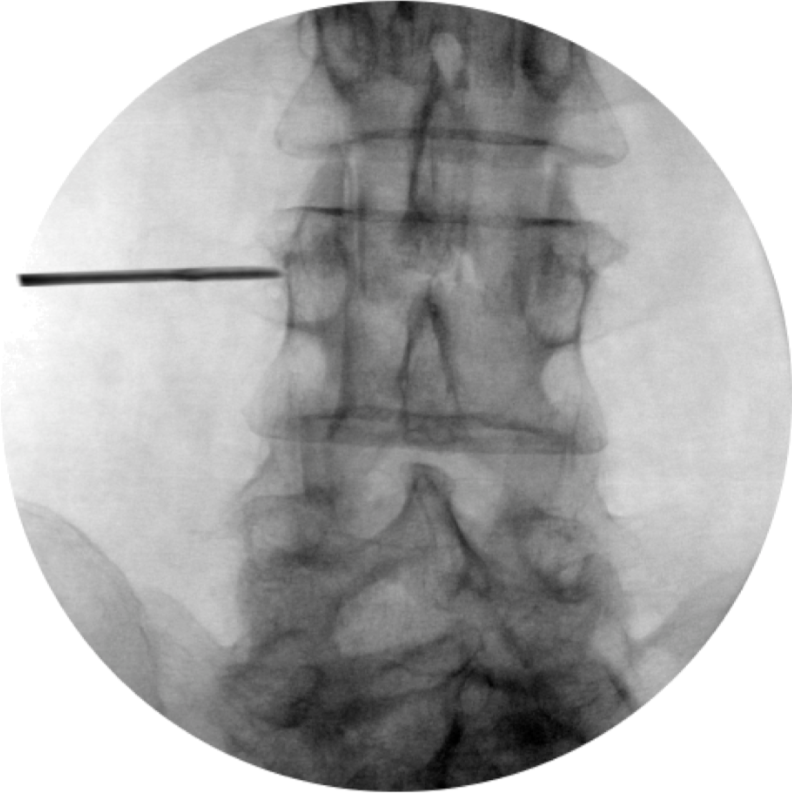
Figure 19b. Lumbar medial branch denervation procedure. AP view of needle placed at junction of SAP and transverse process
Permission to publish granted from Kimberly Clark
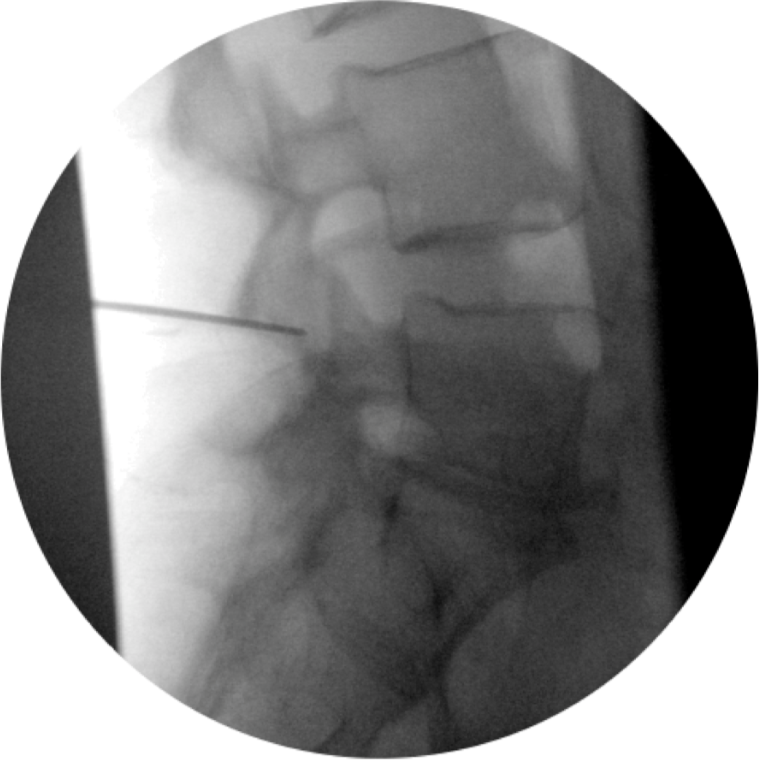
Figure 19c. Lumbar medial branch denervation procedure. Lateral view of needle placed posterior to the foramen
Permission to publish granted from Kimberly Clark
Lesion Size
Lesion size and thus efficacy of RF may also be influenced by preinjection of small volumes of different fluids. Provenzano et al demonstrated that increasing concentrations of NaCl significantly increased lesion size by decreasing impedance around the needle and increasing power output.[51] More studies are needed to determine the optimal preinjection solution and volume.
Complications
There are a number of complications association with RF. Damage to the ventral nerve root is the most feared risk from RF denervation and is the reason for motor stimulation prior to radiofrequency lesioning. Increased pain and post-denervation neuritis are potential complications and may be minimized by injecting steroid (about 5 mg of particulate steroid (i.e. Depo-medrol) along with the LA prior to denervation. Some patients describe transient numbness or dysesthesias, which are usually minor and selflimiting.48 Gazelka H et al found the incidence rate of occipital neuralgia after RFA of the C2-3 facet joint or third occipital nerve to be 19%. This neuralgia is also self limiting, however patients should be advised of its risk prior to the procedure.[65] Rarely, breaks in insulation or equipment malfunction can lead to burns.[14][49] Infections are rare.[50]
Surgery
Surgery has been used to treat facetogenic pain; however, surgery is not recommended for the treatment of facet pain.
Emerging Technology
Magnetic resonance guided focused ultrasound may offer a non-invasive technique to treat facet joint pain. Preliminary studies suggest good efficacy and safety of this technique. This technology may ultimately offer patients a treatment that is non-invasive and free of radiation.[56]
Ultrasound guided medial branch blocks
Ultrasound is also being used to perform medial branch blocks in both the cervical and lumbar regions. Please refer to the ultrasound guided axial blocks section in the pain resource center for a more detailed discussion of this topic.
Summary
Pain arising from the facet joints is a common source of pain and disability, usually due to chronic degeneration. There are no findings in history or physical examination that are pathognomonic for diagnosis, but clinical assessment is important for ruling out other sources of pain and selecting candidates for interventions. Intra-articular or MBBs remain the “gold standard” for diagnosis, but are characterized by a high false-positive rate and lack of specificity. RF denervation can provide good relief in well-selected patients for 6 months to 1 year.
References
- Walker BF: The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J Spinal Disord 2000;13:205-17.
- Cote P, Cassidy JD, Carroll LJ, et al: The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain 2004;112:267-73.
- The Burden of Musculoskeletal Disease in the United States. Rosemont, IL, American Academy of Orthopaedic Surgeons, 2008
- Manchikanti L, Singh V, Pampati V, et al: Analysis of growth of interventional techniques in managing chronic pain in the Medicare population: a 10-year evaluation from 1997 to 2006. Pain Physician 2009;12:9-34.
- Brummett CM, Cohen SP: Facet blocks: Facet joint injections, medial branch blocks, rhizotomy. In Raj's Practical Management of Pain, 4th Edition. Edited by Benzon HT, Rathmell JP, Wu CL, Turk DC, Argoff CE. New York, Elsevier-Mosby, 2006
- Dreyfuss P, Schwarzer AC, Lau P, et al: Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomography study. Spine (Phila Pa 1976) 1997;22:895- 902.
- Chua WH, Bogduk N: The surgical anatomy of thoracic facet denervation. Acta Neurochir (Wien) 1995;136:140-4.
- Barnsley L, Lord S, Bogduk N: Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain 1993;55:99-106.
- Cohen SP, Raja SN: Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology 2007;106:591-614.
- Panjabi MM, Oxland T, Takata K, et al: Articular facets of the human spine. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976) 1993;18:1298-310.
- Lord SM, Barnsley L, Wallis BJ, et al: Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine (Phila Pa 1976) 1996;21:1737-44
- Lord SM, Barnsley L, Wallis BJ, et al: Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med 1996;335:1721-6.
- Chow DH, Luk KD, Evans JH, et al: Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine (Phila Pa 1976) 1996;21:549-55.
- Oudenhoven RC: Lumbar monoradiculopathy due to unilateral facet hypertrophy. Neurosurgery 1982;11:726-7.
- Manchikanti L, Boswell MV, Singh V, et al: Prevalence of facet joint pain in chronic spinal pain of cervical, thoracic, and lumbar regions. BMC Musculoskelet Disord 2004;5:15.
- Cohen SP: Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg 2005;101:1440-53.
- Helbig T, Lee CK: The lumbar facet syndrome. Spine (Phila Pa 1976) 1988;13:61-4.
- Cohen SP, Bajwa ZH, Kraemer JJ, et al: Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Reg Anesth Pain Med 2007;32:495-503.
- Cohen SP, Stojanovic MP, Crooks M, et al: Lumbar zygapophysial (facet) joint ra- diofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. Spine J 2008;8:498-504.
- Murtagh FR: Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine (Phila Pa 1976) 1988;13:686-9.
- Marks RC, Houston T, Thulbourne T: Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain 1992;49:325-8.
- Rathmell JP: Atlas of Image-Guided Intervention in Regional Anesthesia and Pain Medicine. Philadelphia, Lippincott Williams and Wilkins, 2005
- International Spine Intervention Society: Practice Guidelines-Spinal Diangostic and Treatment Procedures, ISIS, 2004
- Barnsley L, Lord S, Wallis B, et al: False-positive rates of cervical zygapophysial joint blocks. Clin J Pain 1993;9:124-30.
- Lord SM, Barnsley L, Bogduk N: The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain 1995;11:208-13.
- Barnsley L, Lord SM, Wallis BJ, et al: The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine (Phila Pa 1976) 1995;20:20-5
- Cohen SP, Strassels SA, Kurihara C, et al: Randomized study assessing the accuracy of cervical facet joint nerve (medial branch) blocks using different injectate volumes. Anesthesiology 2010;112:144-52.
- Dory MA: Arthrography of the lumbar facet joints. Radiology 1981;140:23-7.
- Moran R, O'Connell D, Walsh MG: The diagnostic value of facet joint injections. Spine (Phila Pa 1976) 1988;13:1407-10.
- Hogan QH, Abram SE: Neural blockade for diagnosis and prognosis. A review. Anesthesiology 1997;86:216-41.
- Lee CJ, Kim YC, Shin JH, et al: Intravascular injection in lumbar medial branch block: a prospective evaluation of 1433 injections. Anesth Analg 2008;106:1274-8.
- Kaplan M, Dreyfuss P, Halbrook B, et al: The ability of lumbar medial branch blocks to anesthetize the zygapophysial joint. A physiologic challenge. Spine (Phila Pa 1976) 1998;23:1847-52.
- Stojanovic MP, Sethee J, Mohiuddin M, et al: MRI analysis of the lumbar spine: can it predict response to diagnostic and therapeutic facet procedures? Clin J Pain 2010;26:110-5.
- Barnsley L, Lord SM, Wallis BJ, et al: Lack of effect of intraarticular corticosteroids for chronic pain in the cervical zygapophyseal joints. N Engl J Med 1994;330:104750.
- Carette S, Marcoux S, Truchon R, et al: A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med 1991;325:1002-7.
- Manchikanti L, Singh V, Falco FJ, et al: Cervical medial branch blocks for chronic cervical facet joint pain: a randomized, double-blind, controlled trial with one-year follow-up. Spine (Phila Pa 1976) 2008;33:1813-20.
- Gallagher J, Petriccione di Vadi PL, Wedley JR, et al: Radiofrequency facet joint denervation in the treatment of low back pain: A prospective controlled double-blind study to assess its efficacy. Pain Clinic 1994;7:193-98.
- King JS, Lagger R: Sciatica viewed as a referred pain syndrome. Surg Neurol 1976;5:46- 50.
- Leclaire R, Fortin L, Lambert R, et al: Radiofrequency facet joint denervation in the treatment of low back pain: a placebo-controlled clinical trial to assess efficacy. Spine (Phila Pa 1976) 2001;26:1411-6
- Sanders M, Zuurmond WW: Percutaneous intra-articular lumbar facet joint denervation in the treatment of low back pain: A comparison with percutaneous extra-articular lumbar facet denervation. Pain Clinic 1999;11:329-35.
- Stovner LJ, Kolstad F, Helde G: Radiofrequency denervation of facet joints C2-C6 in cervicogenic headache: a randomized, double-blind, sham-controlled study. Cephalalgia 2004;24:821-30.
- van Kleef M, Barendse GA, Kessels A, et al: Randomized trial of radiofrequency lumbar facet denervation for chronic low back pain. Spine (Phila Pa 1976) 1999;24:1937-42.
- van Wijk RM, Geurts JW, Wynne HJ, et al: Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion- controlled trial. Clin J Pain 2005;21:335-44.
- Farrar JT, Young JP, Jr., LaMoreaux L, et al: Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149-58.
- Dreyfuss P, Halbrook B, Pauza K, et al: Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976) 2000;25:1270-7.
- McDonald GJ, Lord SM, Bogduk N: Long-term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery 1999;45:61-7; discussion 67-8.
- Schofferman J, Kine G: Effectiveness of repeated radiofrequency neurotomy for lumbar facet pain. Spine (Phila Pa 1976) 2004;29:2471-3.
- North RB, Han M, Zahurak M, et al: Radiofrequency lumbar facet denervation: analysis of prognostic factors. Pain 1994;57:77-83.
- Ogsbury JS, 3rd, Simon RH, Lehman RA: Facet "denervation" in the treatment of low back syndrome. Pain 1977;3:257-63.
- Cheng J, Abdi S: Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag 2007;11:141-147.
- Martin BI, Deyo RA., et al. Expenditures and health care status among adults with back and neck problems. JAMA 2008; 299:656-64. Erratum in: JAMA 2008; 299:2630.
- Manchikanti L, Pampati V, et al. Growth of spinal interventional pain management techniques: Analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976) 2013; 38: 157-168.
- Manchikanti L. Pampati M. et al, Assessment of the escalating growth of facet joint interventions in the medicare population in the United States from 2000 to 2011. Pain Physician 2013; 16: E365-E378.
- Matar H., Navalkissoor S. Is hybrid (SPECT/CT) a useful adjunct in the management of suspected facet joints arthropathy. International Orthopedics 2013; 37(5): 865-70.
- MA K. Yiqun M. Efficacy of diclofenac sodium in pain relief after conventional radiofrequency denervation for chronic facet joint pain: a double-blind randomized controlled trial. Pain Medicine 2011; 12(1): 27-35.)
- Weeks EM, Platt MW. MRI-guided focused ultrasound to treat facet joint osteoarthritis low back pain-case series of an innovative new technique. Eur Radiol 2012; 22(12): 2822-35.
- Provenzano D. Leibert M. Increasing the NaCl concentration of preinjected solution enhances monopolar radiofrequency lesion size. Regional Anesthesia and Pain Medicine 2013; 38(2): 112-23
- Pearson AM, Ivancic PC et al. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. @004; 29: 390-7
- Winklestein BA, McLendon Re. et al. An anatomical investigation of the human cervical facet capsule, quantifying muscles insertion area. J Anat. 2001; 198:45-61
- Siegmund GP, Myers BS, et al. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine. 2001; 26:2095-101.).
- Dwer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns. I: a study in normal volunteers. Spine. 1990; 15:453-7.
- Cohen S, Williams K et al. Multicenter, Randomized, Comparitive Cost-effectiveness Study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology 2010; 113:395- 405
- Zan Zundert, J., Mekhail N, Canelderen P. Diagnostic medial branch blocks before lumbar radiofrequency zygapophysial (facet) joint denervation benefit or burden. Anesthesiology 2010; 113: 276-8.)
- Rambaransingh B, Standford G, Burnham R. The effect of repeated zygapophysial joint radiofrequency neurotomy on pain, disability, and improvement duration. Pain Med. 2010; 11:1343-7.)
- Gazelka H, et al. Incidence of neuropathic pain after radiofrequency denervation of the third occipital nerve. J of Pain Research 2014; 7: 195-98.)
- Cohen S. et al, Does sensory stimulation threshold affect lumbar facet radiofrequency denervation outcomes? A prospective clinical correlational study. Anesthesia-Analgesia 2011; 113(5): 1233-41.
Leave a commentOrder by
Newest on top Oldest on top